Please refer to the first paragraph of the blog post titled “Mutations in the ECD of Zip4 lead to misfolding of Zip4 and acrodermatitis enteropathica (AE)” on the “Metals, Metalloproteins and Disease” blog for a description of acrodermatitis enteropathica (AE), its causes, its symptoms, and its traditional treatments.
In brief, this paper explores a potential new treatment option for patients with AE. Instead of pure/free zinc (Zn) uptake, they wondered if Zn attached to a given amino acid may be uptook by proteins that bring AAs into the cell. They found that the answer was a resounding yes, albeit it was done slower than uptake of free Zn by Zn importers (Fig1. A,B,C,D,E,F,G,H). However, when given sufficient time, the same amount of Zn taken into the cell between free Zn and ZnAAs by their respective importers was comparable (Fig1. A,B,C,D,E,F,G,H).
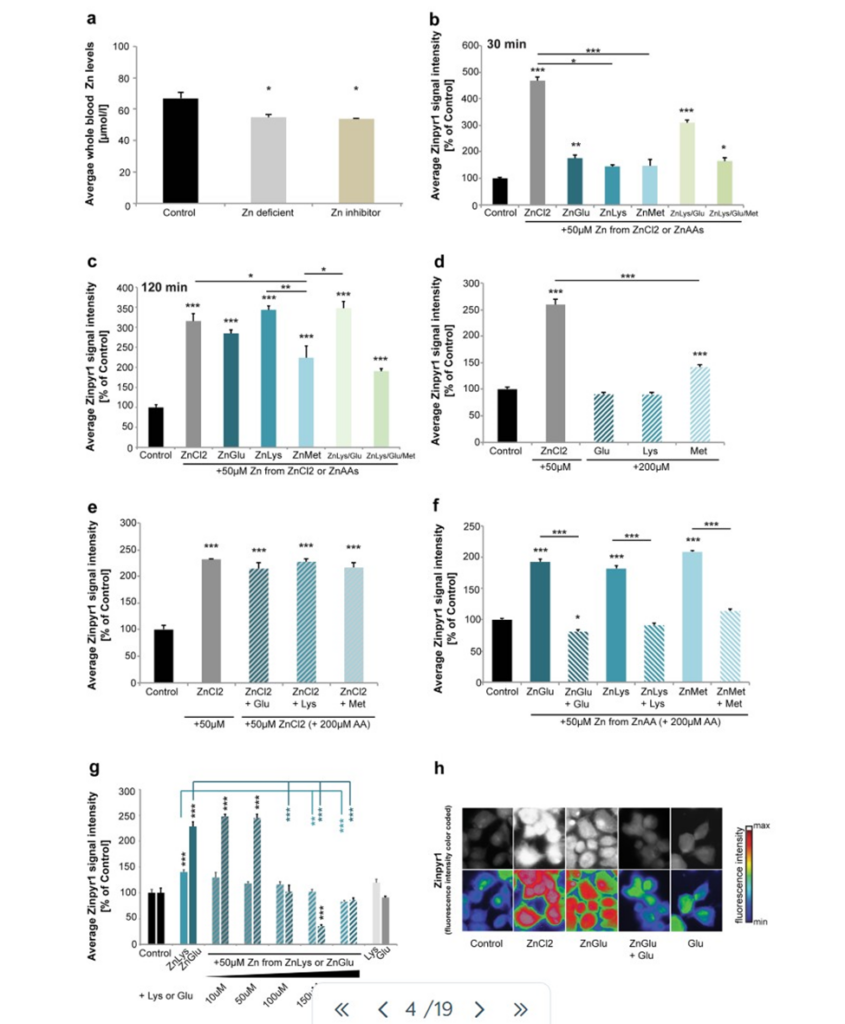
Figure 1: A measures the amount of free Zn in the blood in three different diets, a normal diet, Zn deficient diet, and a normal diet with Zn inhibitors. B-H measure Zn uptake in varying conditions and times of ZnAAs and free Zn, which demonstrates that after a certain amount of time, Zn becomes present in the cell in similar quantities.
Next, researchers wanted to determine whether patients with AE could use AA transporters to get Zn into their cells by taking in Zn that is attached to AAs (Fig2. B-D, G-H). Again, the researchers found that it is possible (Fig2. B-D, G-H). However, the presence of more Zn in the body is required since normal AAs will compete for uptake by the AA receptors. Additionally, the researchers found no evidence that the uptake of AAs are mediated in anyway, meaning that it is theoretically possible that more Zn than is needed could be taken in, if someone’s diet is unbalanced, for example.
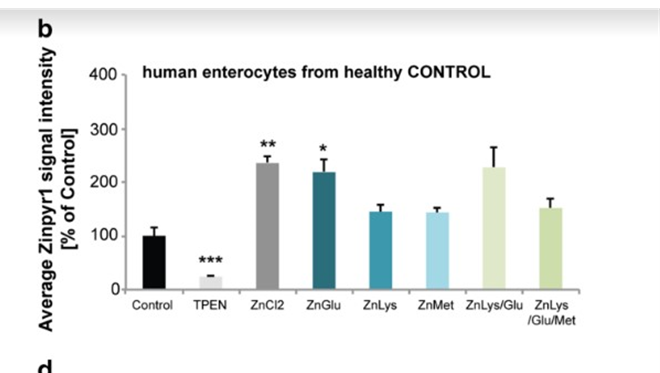
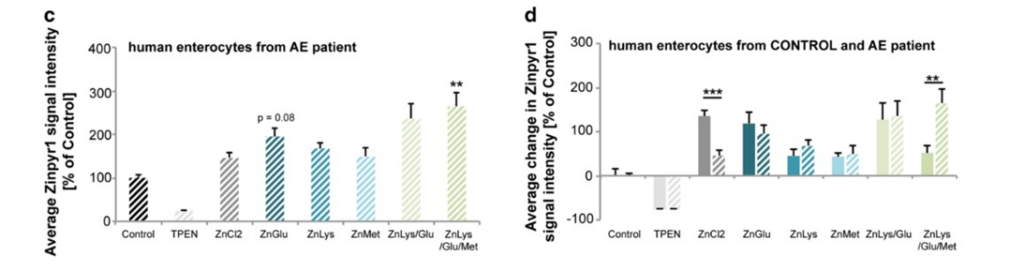
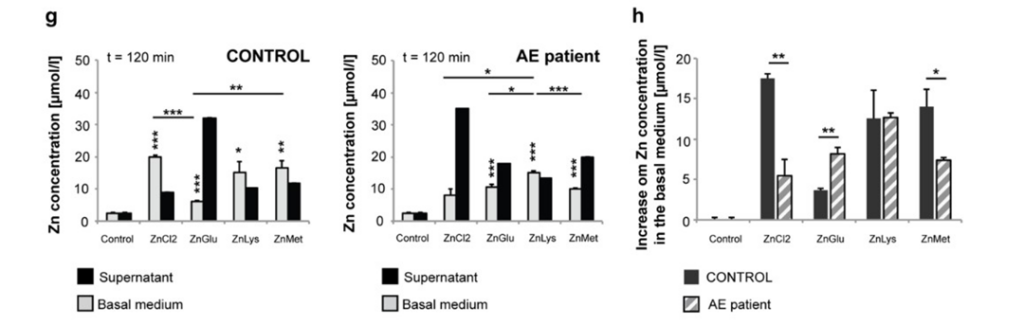
Figure 2: B shows the amount of uptake of various Zn conditions in healthy people. C explores how much Zn is brought into the cell in various Zn conditions in AE patients. D shows the comparison of B and C. G depicts the amount of Zn that the enterocyte was able to move into the basal medium from the liquid (supernatant) in both healthy people and AE patients. H demonstrates the comparison between the two graphs in G.
This data leads to some main conclusions from this paper. Although free Zn must enter the cell through normal Zn transporters, ZnAAs can be imported by AA importers, but normal AAs will compete for the ability to use these transporters with ZnAAs. ZnAAs, if given enough time to enter the cell, will lead to a similar amount of Zn entering the cell as when free Zn is used. There is no mechanism that regulates how much Zn can be brought into the cell when ZnAAs are imported into the cell. In the future, supplementation with ZnAAs or diet changes to include more ZnAAs may be a good treatment alternative to pure free Zn for people with AE, since being on pure Zn supplements may have unknown detrimental effects on the patient.
Citations:
Sauer, Ann Katrin. “Characterization of Zinc Amino Acid Complexes for Zinc Delivery in Vitro Using Caco-2 Cells and Enterocytes from Hipsc.” Biometals : An International Journal on the Role of Metal Ions in Biology, Biochemistry, and Medicine, U.S. National Library of Medicine, 17 July 2017, pubmed.ncbi.nlm.nih.gov/28717982/. Accessed 26 Sept. 2024.


