Metals are essential for the normal activity of all living beings. Besides their involvement in oxygen storage and role in many essential proteins, they are also utilized by the immune system to kill invading pathogens. While a sustained diet of metals is necessary, an overaccumulation of metals can lead to errant protein function and DNA damage. Human immune cells leverage these dynamics by either restricting metals from pathogens or overloading pathogens with metals, with each process leading to pathogen death. In order to survive in the human body, pathogens must in turn find ways to either acquire metals to allow for normal cellular processes or export away metals to prevent their toxicity. This back-and-forth between host metal deprivation/intoxication and pathogen metal scavenging/export has been labelled as “nutritional immunity”.
SLC11A1, formerly known as NRAMP1, is a host protein involved in metal transport that has been implicated in multiple disease types. Mutations in this gene have been associated with increased susceptibility to infectious disease, and overexpression of the protein has been predictive of cancer patient prognosis. Initial studies demonstrated that knockout of SLC11A1 in immune cells decreased killing of pathogens and resulted in more potent infections, suggesting that the protein is involved in pathogen removal. Since then, however, the specific function of the protein has been a topic of debate. Some theorized that the transporter kills pathogens by overloading them with metals, while others theorized that the protein deprives pathogens of the metals necessary for normal function. Moreover, it is possible that the downstream immune cell-activating effects induced by SLC11A1 are more influential in pathogen killing than its metal transport functions.
In this study, Dr. Dirk Bumann and colleagues looked to specify the effector function of SLC11A1 against Salmonella infection by removing SLC11A1 from mice (referred to as a knock-out, or KO). By assessing the survival of a portfolio of Salmonella mutants lacking metal import or export mechanisms, they looked to elucidate whether Iron, Manganese, Magnesium, or Zinc transport is essential for SLC11A1-mediated killing, and whether the host protein looks to prevent pathogens from acquiring metals or overload them with metals, causing toxicity.
The authors first injected Salmonella into SLC11A1-expressing or SLC11A1 KO mice, subsequently determining the protein profile of such Salmonella to determine how Salmonella react to the presence of host SLC11A1. They determined that Salmonella metal uptake mechanisms are prioritized in the presence of SLC11A1, while mechanisms to evade other host immune cell tactics are similar with or without host SLC11A1. This suggested that SLC11A1 is a metal deprivation mechanism utilized by host cells to kill Salmonella.
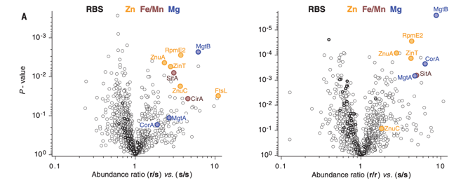
Figure 1. Metal uptake mechanisms are upregulated in Salmonella following incubation in SLC11A1-expressing mice. Values greater than 1 indicate increased expression of the protein in Salmonella following inoculation in SLC11A1-expressing mice when compared to SLC11A1-deficient mice.
To build on these observations, the authors then produced a produced a portfolio of mutant Salmonella lines, each with a single metal import mechanism removed. The authors injected these mutants into SLC11A1-expressing or SLC11A1-deficient mice. Of the mutants, the authors observed that mutant Salmonella lines lacking the MgtB protein, an aggressive transporter involved in the acquisition of the essential metal magnesium, were less likely to survive when injected into mice expressing SLC11A1. In SLC11A1-deficient mice, this mutant survived and caused a more substantial infection. This finding suggests that SLC11A1 deprives pathogens of magnesium to disrupt normal function, and that Salmonella metal acquisition mechanisms are essential when host SLC11A1 is present.
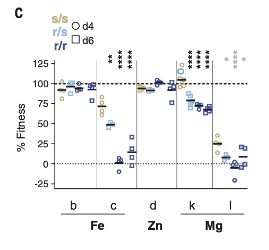
Figure 2. Mutant k lacking the Magnesium transporter MgtB have reduced fitness in the presence of SLC11A1. Mutant l lacks both MgtB and MgtA, another magnesium transport mechanism.
Without any active deprivation of magnesium, even Salmonella lacking aggressive magnesium acquisition mechanisms, such as MgtB, can survive, likely due to other magnesium acquisition methods and greater magnesium availability in the host. In an attempt to induce Salmonella magnesium deprivation without SLC11A1, the authors then independently deprived MgtB KO Salmonella, injected into in SLC11A1-deficient mice, of Magnesium. To accomplish this, they engineered a mutant Salmonella line with significantly hindered, but not completed removed, magnesium acquisition abilities. By doing this, they hoped to imitate the effect of SLC11A1, reducing the “availability” of magnesium in the host. When grown in SLC11A1-deficient mice, this mutant Salmonella line had a similar need for the aggressive Salmonella magnesium acquisition protein MgtB as normal Salmonella grown in mice with intact SLC11A1. Furthermore, these Salmonella were similarly unable to cause infection. This further implicates magnesium restriction from pathogens as the effector mechanism of host SLC11A1, which reduces the availability of magnesium for pathogen usage.
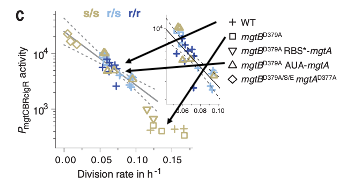
Figure 3. Partial MgtB/MgtA DKO Salmonella have similar survival rates and mgtB promoter activity in SLC11A-deficient mice to WT Salmonella inoculated in mice with intact SLC11A1. Normally, MgtB KO lines proliferate freely and require little magnesium import in SLC11A1-deficient mice. When magnesium uptake mechanisms are partially eliminated, however, division rate is reduced and mgtB promoter activity is increased, equivalent in effect to WT Salmonella being deprived of Magnesium by SLC11A1.
Altogether, these results indicate that SLC11A1 deprives magnesium from Salmonella, which require increased magnesium uptake mechanisms to survive. These results elucidate the role of a commonly debated protein, adding to the catalogue of nutritional immune defense proteins that protect against extracellular pathogens and the counterpunching function of pathogen proteins that circumvent host metal deprivation. However, the differences in SLC11A1 expression profiles amongst host cell subsets are yet to be elucidated and may provide further detail regarding the specifcities of SLC11A1-mediated elimination of pathogens.
References:
Cunrath & Bumann, 2019. Host resistance factor SLC11A1 restricts Salmonella growth through magnesium deprivation. Science 366,995-999. DOI:10.1126/science.aax7898


