Copper is an essential nutrient for all living beings. However, accumulating excess copper can lead to toxicity due to its highly reactive nature. In humans, immune cells leverage this toxicity to kill pathogens, transporting excess copper to pathogens and inducing copper-based killing.
Macrophages are an immune cell subset involved in the killing of extracellular pathogens. Following recognition of an invader, macrophages will engulf the threat and form an isolated intracellular compartment, or phagosome, around the pathogen. Toxic molecules are then exported into the phagosome to swiftly eliminate the invader. In their phagosomes, macrophages utilize the copper transport protein ATP7A to flood pathogens with copper, leading to the subsequent production of toxic elements and disruption of normal function in the pathogen. This process has been labelled “copper intoxication”. To circumvent copper intoxication, pathogens have developed modes of copper tolerance, including proteins such as CopA and GolT in Salmonella, which export copper to prevent excess accumulation.
In the current study, the Micheal J. Petris and colleagues looked to demonstrate the necessity of ATP7A for copper intoxication mechanisms in macrophage phagosomes, subsequently investigating the activity of Salmonella metal exporters CopA and GolT in response to ATP7A. They utilize a mouse system in which the ATP7A gene is selectively removed in myeloid cells, such as macrophages, as well as Salmonella lines containing mutant (also labelled as knockout, or KO) CopA and GolT genes.
To demonstrate that macrophage activation leads to ATP7A trafficking to the phagosome, the authors first “fed” macrophages heat-killed, fluorescent E. Coli, allowing for identification of phagosomes. When utilized in tandem with a fluorescent ATP7A label, the authors showed that, following activation, ATP7A localizes in the phagosome.
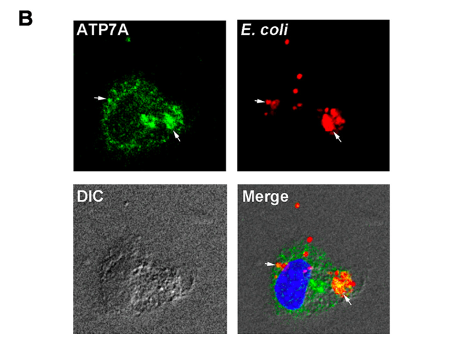
Figure 1. LPS and IFN-g activation stimulates ATP7A trafficking to the phagosome.
The authors then assessed the survival rate of Salmonella in ATP7A KO macrophages. The authors assess the colony growth of Salmonella following incubation in either ATP7A KO or ATP7A-expressing macrophages, confirming that Salmonella have increased potential for survival in ATP7A KO macrophages when compared to ATP7A-expressing macrophages.
The authors also assessed the copper-transporting function of ATP7A by incubating Salmonella on Cu-deficient media prior to incubation in macrophages. Utilizing a fluorescent dye to identify intracellular copper within the salmonella-fed macrophages, the authors show that ATP7A KO macrophages demonstrate a reduced capacity to transport copper into phagosomes.
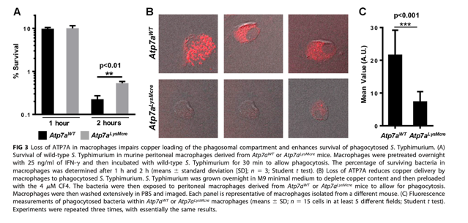
Figure 2. ATP7A KO in macrophages results in increased Salmonella survival and reduced copper delivery into phagosomes.
In response to host metal intoxication mechanisms, pathogens have evolved mechanisms to export copper from their vulnerable intracellular compartments. In Salmonella, these include the copper exporters CopA and GolT. To investigate the activity of these transporters in response to macrophage ATP7A, The authors utilized a CopA and GolT double knock-out (DKO) salmonella line to assess the necessity of these proteins in the presence of macrophage ATP7A. Within macrophages containing intact ATP7A, CopA/GolT DKO Salmonella demonstrate reduced capability for survival when compared to Salmonella containing intact CopA and GolT. When Salmonella are incubated in ATP7A KO macrophages, this difference cannot be observed– CopA/GolT DKO Salmonella demonstrate equal survival when compared to normal salmonella.
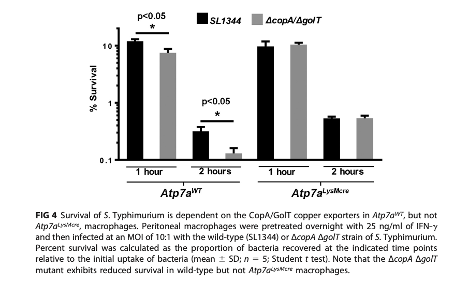
Figure 3. CopA/GolT Salmonella demonstrate decreased survival only in macrophages with intact ATP7A.
Lastly, the authors assessed the survival capabilities of CopA/GolT Salmonella and normal Salmonella in mice containing ATP7A KO macrophages or mice with intact ATP7A. The authors demonstrate that CopA/GolT Salmonella are less likely to survive than WT Salmonella only in mice with intact macrophage ATP7A. In mice without ATP7A expressed in their macrophages, CopA/GolT and WT Salmonella are equally as likely to survive.
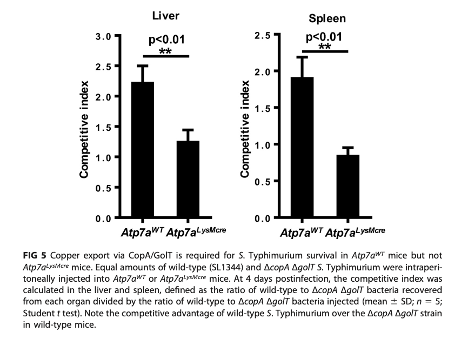
Figure 4. CopA/GolT DKO Salmonella demonstrate reduced survival capabilities than normal salmonella only in mice containing intact macrophage ATP7A. Competitive index: a value greater than 1 indicates that normal Salmonella are more prevalent than DKO Salmonella following mouse inoculation.
In conclusion, the authors demonstrate that ATP7A is necessary for copper-mediated killing of Salmonella in macrophages, providing a necessary contribution to elimination of bacterial pathogens. Opposing copper-exporting ATPases GolT and CopA provide a necessary mode of defense for Salmonella against such copper intoxication. These results provide a canonical demonstration of nutritional immunity, detailing a mechanism by which immune cells utilize the reactive properties of metals to kill pathogens and a modes of defense employed by pathogens to circumvent metal-mediated killing.
References:
Ladomersky et al., 2017. Host and Pathogen Copper-Transporting P-Type ATPases Function Antagonistically during Salmonella Infection. Infection and Immunity. https://doi.org/10.1128/iai.00351-17


