Manganese is an essential nutrient attained through diet. While manganese is a necessary nutrient, it is toxic in excess. An excess of Manganese can result in a neurodegenerative disease called manganism. Manganism is generally the result of excessive manganese inhalation, mostly in work environments. However, in 2012 an inherited case of Slc30a10 mutation was identified in a case of manganese excess was discovered prompting a series of research studies into the effects of dysfunctional manganese transporters. This article researches the effects on manganese homeostasis when knocking out Slc30a10, a metal transporter in both the liver and the stomach in mice.
The first area the paper studies is total knockout of Znt10 (Slc30a10) in mice. They found that complete knockout results in a capitulation of manganism. Meaning excess manganese found in liver, bone, brain and the duodenum (Fig. 1). They also found an increase in red blood cell count which may hint at another disease resulting from manganese excess, polycythemia (a red blood cell cancer).
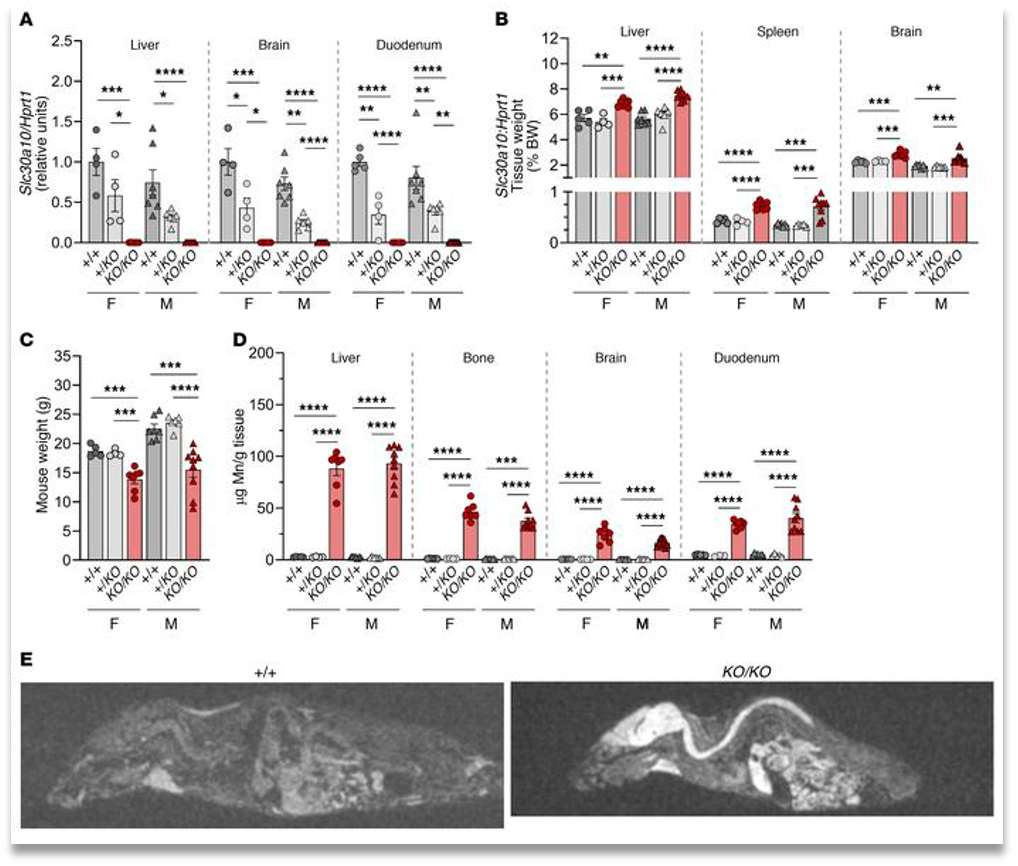
They then continued the study by introducing high manganese intake in the mice and compared them to the wild type. Finding that while Mn (manganese) levels were slightly increased in tissues except for in the brain. It also increased hepatobiliary excretion of Mn and lowered the iron levels in tissues showing that Mn can act as a competitive inhibitor.
The next part of the study will study localized knockout in either the enterocytes or the hepatocytes to see if this would result in a manganese excess that capitulates with that of manganism.
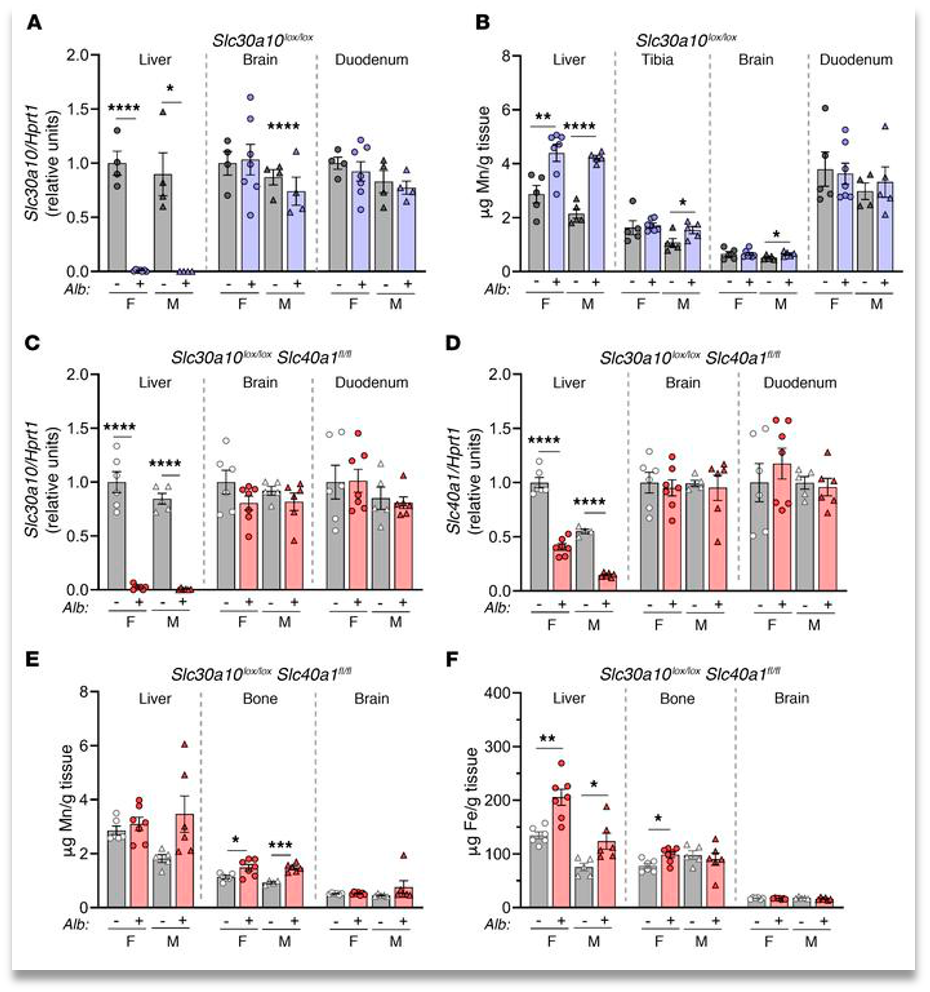
They first studied localized knockout of Znt10 in the hepatocytes. The results were that there was only a very slight increase in Mn excess in the blood, brain, and bone. They also found only a 2x increase in levels within the hepatocytes. However, it does completely shut down the excretion of manganese through bile.This shows that there can be an uptake in Mn excretion through alternate routes.(fig. 2)
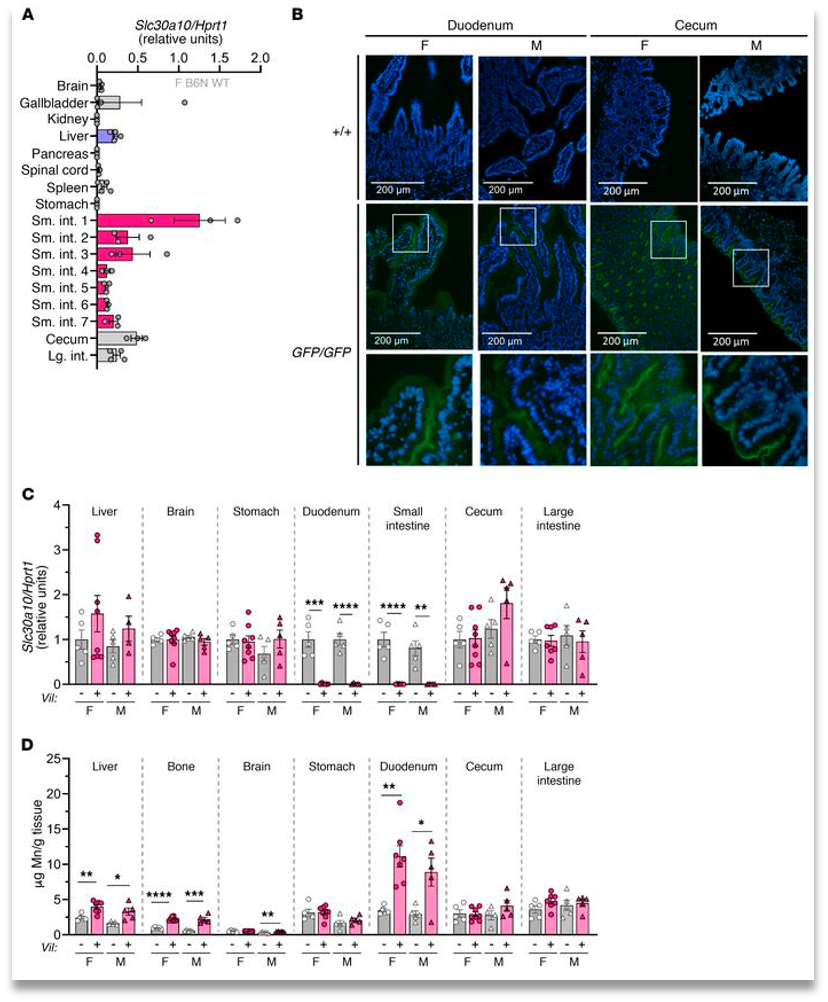
Enterocyte inactive Znt10 leads to a slight increase in manganese levels in the liver, bone, and intestines. While showing a large increase in the duodenum, but no increase in brain levels(Fig. 3). This once again shows that while the systemic release of Mn is important, it’s inactivity does not cause a severe Mn excess throughout the body also hinting at a different excretion system being utilized.
The consistent manganese levels in the brain throughout the experiments (except for total body knockout of Znt10) led them to believe that there was crossover between systemic and hepatobiliary excretion of manganese. The next logical step was to knockout Znt10 in both the enterocytes and hepatocytes. They found that knocking out the transporters in both of these areas led to a large increase in manganese in everywhere but the brain and it led to no increase in red blood cell count. (Fig. 4)
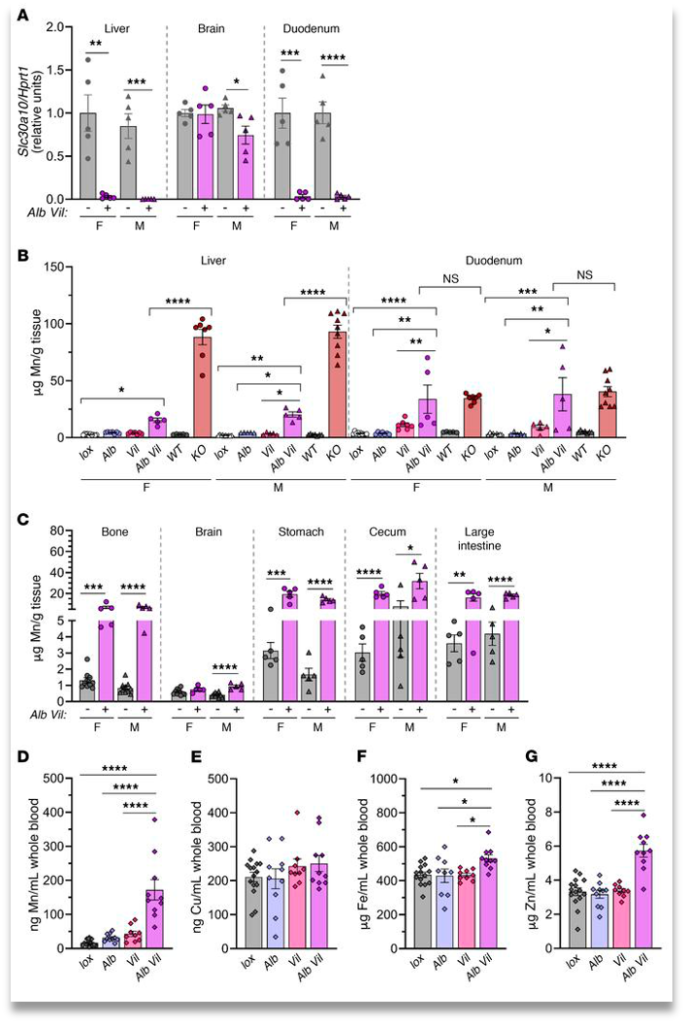
In conclusion, while total knockout of Slc30a10 capitulates with manganism, localized knockouts do not. This shows that when one route of excretion (hepatobiliary or systemic) is disabled, the other can uptake more manganese to keep homeostasis. However, when both routes are disabled, Mn excess in the brain is still at functional levels hinting at there being another route of manganese excretion that is currently unknown.
Citation: •Mercadante, Courtney J., et al. “Manganese Transporter SLC30A10 Controls Physiological Manganese Excretion and Toxicity.” The Journal of Clinical Investigation, American Society for Clinical Investigation, 2 Dec. 2019, https://www.jci.org/articles/view/129710#sd.


