Alzheimer’s Disease (AD) is a severe neurodegenerative disease that is marked by the buildup and accumulation of toxic waste in the central nervous system. It is the most common cause of dementia and has one of the highest cases of death in America with no known cure. The progression of the disease has an early pre-symptomatic stage marked by short-term memory loss undetectable through testing before progressing to a mild-to-moderate symptomatic stage characterized by mood swings and moderate memory loss until reaching a severe stage of the disease marked by severe memory loss, motor impairments, and/or struggles with communication. AD is known to most significantly affect memory domains in the brain which is why the Hippocampus, the brain region most associated with learning and memory, is the structure most severely affected in Alzheimer’s Disease progression.
On a cellular level, AD is characterized by a lack of proper metabolic clearance and subsequent accumulation of neuritic plaques and protein aggregates. APP is one of the two hallmark protein aberrations in AD. APP is a precursor protein for Aß synthesis which is a waste product that begins to aggregate in AD. Aß is ubiquitously expressed around the body, but in AD, will start to form oligomers which are thought to be the most toxic forms of Aß. The other cellular hallmark in AD are neurofibrillary tangles (NFTs) which are aggregated forms of an essential protein in neurons, tau. Tau is an indispensably important protein that is critical for maintaining the structural stability throughout the axon of neurons where the cellular signal is propagated to other neurons. In AD tau becomes hyperphosphorylated in response to damage or improper protein folding and begins to stick with other tau and aggregate into toxic tangles.
In recent studies, these Aß oligomers and NFTs have been shown to signal for the activation of an inflammatory complex responsible for purging sick cells and helping the body to build biological immunity called the NLRP3 Inflammasome (Sharma and de Alba, 2021). The Inflammasome is a multi-unit protein complex containing a highly organized scaffold of NLRP3 monomers to form this large structured multi-domain complex.
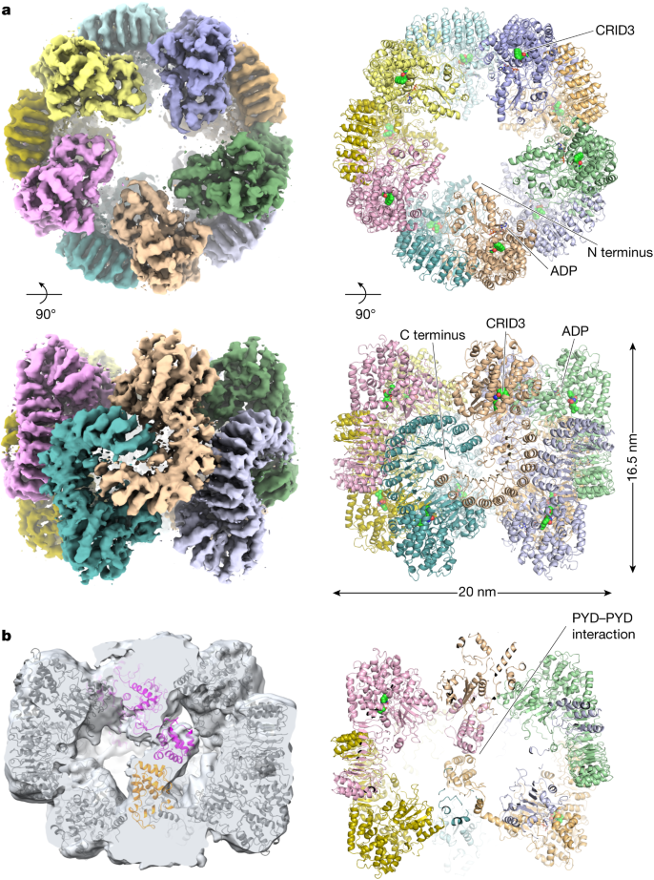
The Inflammasome’s formation is a two-step process: priming, where pathogenic stress causes the activation of specific genes (NF-KB) to promote the synthesis of the NLRP3 monomer and immature cytokines, and activation, where sustained exposure to NFTs or Aß disrupts lysosomal pathways which raises intracellular Ca2+ and causes the formation of the complex (Barczuk et al., 2022).
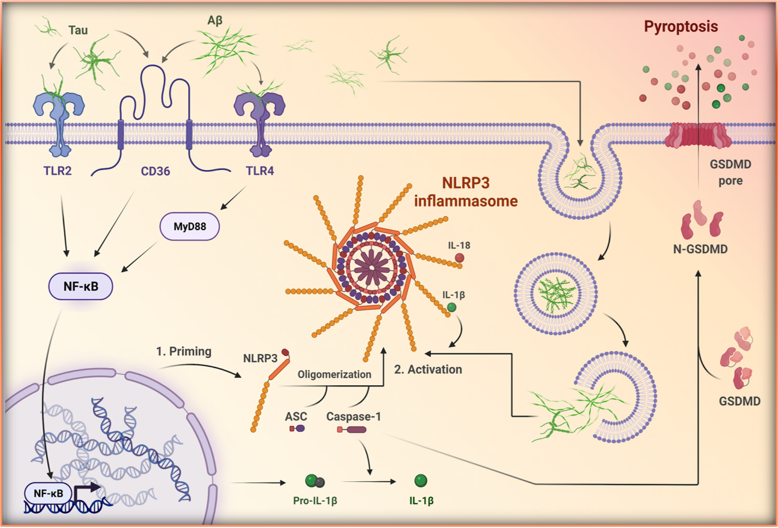
Shown in the figure above describes the AD-specific progression of Inflammasome-mediated cell death. The figure shows Tau in NFTs as well as Aß protein aggregates binding to specific receptors that sense their extracellular environments—In this case TLRs and CD36— and elicit a danger response. This response leads to an inflammatory signal cascade through NF-KB signaling. This signaling then results in downstream production of chemokines ready for cleavage and NLRP3 monomers ready for complex formation. However, once the cell is primed, if any NFTs or Aß plaques are taken into the cell, that will be enough to elicit the NLRP3 Inflammasome’s formation. Once the complex is formed and the NLRP3 oligomerizes, caspase signaling leads to the cleavage of the stored chemokines which result in a form of cell death called pyroptosis, where copious pores form in the cell membrane, and it is mediated by GSDMD. This form of cell death then release all of the stored chemokine within the cell to signal through paracrine and endocrine pathways the same danger signals just received by the recently inflamed cell.
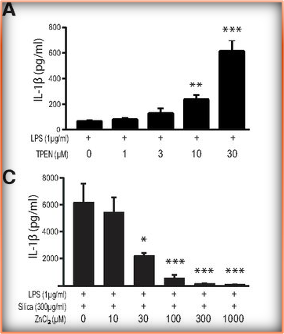
Previous work has demonstrated that there is a relationship between NLRP3-related inflammation and AD progression (Yao et al., 2024). Moreover, emergent work has also been in support of Zn deficiency mediating NLRP3-dependent inflammation through various mechanisms (Coll et al., 2015). Zn in an essential micronutrient involved in inflammatory pathology among other important functions like cell survival or tissue recovery. In inflammation, Zn is frequently used to make chemokines as well as being used to make other proteins during protein synthesis. Due to zinc’s importance, it is a tightly regulated trace element which is swiftly moved in and out of cellular compartments or of the cell by Zn transporters or the Zrt-Irt family. Therefore, adding a Zn chelator such as TPEN in vitro to deplete Zn levels should show an increase in pro inflammatory biomarkers due to this disrupted homeostasis of an essential element. It then stands to reason that Zn supplementation should have the opposite effect. This is precisely what (Rivers-Auty et al., 2021) found by adding the Zn chelator TPEN in increasing concentrations; they found that an essential chemokine which gets created through the NLRP3 NF-KB-dependent pathway was increasing in concentration signifying a greater immune response as less Zn became available. On the other hand, panel C shows Zn supplementation (with ZnCl2) on concentrations of IL-1ß and it demonstrates the inverse relationship because as more Zn is added to cells, there is a diminishing concentration of IL-1ß which meaning less and less of an immune response.
Taken together, data suggest that Zn homeostasis is vital for cognitive well-being and that deficiencies in this crucial micronutrient can lead to inflammatory cognitive outcomes. As more therapeutics are made for NLRP3 and other inflammatory diseases, it is prudent to recognize the importance of Zn for better cognitive outcomes.
References
Barczuk J, Siwecka N, Lusa W, Rozpedek-Kaminska W, Kucharska E, Majsterek I (2022) Targeting NLRP3-Mediated Neuroinflammation in Alzheimer’s Disease Treatment. Int J Mol Sci 23.
Coll RC et al. (2015) A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21:248-255.
Rivers-Auty J, Tapia VS, White CS, Daniels MJD, Drinkall S, Kennedy PT, Spence HG, Yu S, Green JP, Hoyle C, Cook J, Bradley A, Mather AE, Peters R, Tzeng TC, Gordon MJ, Beattie JH, Brough D, Lawrence CB (2021) Zinc Status Alters Alzheimer’s Disease Progression through NLRP3-Dependent Inflammation. J Neurosci 41:3025-3038.
Sharma M, de Alba E (2021) Structure, Activation and Regulation of NLRP3 and AIM2 Inflammasomes. Int J Mol Sci 22.
Yao J, Sterling K, Wang Z, Zhang Y, Song W (2024) The role of inflammasomes in human diseases and their potential as therapeutic targets. Signal Transduct Target Ther 9:10.