Cobalt is an essential metal which is crucial for many bodily functions, primarily neurological functions (relating to the nervous system) and hematological functions (relating to the blood). Interestingly, unlike metals such as zinc, cobalt is not naturally produced in the body. So, how is cobalt brought into the body? Cobalt is generally consumed in the form of vitamin B12 (also known as cobalamin) from sources like meat and dairy. As mentioned before, cobalt assists in neurological and hematological functions, and these benefits are largely derived from the intake of vitamin B12. However, issues can arise when cobalt is either deficient or in excess. Deficiency is typically the result of insufficient vitamin B12 levels in the body, either due to insufficient intake of vitamin B12, or an underlying condition impairing the body’s ability to absorb vitamin B12 into the bloodstream. These deficiencies can be treated by supplementation of vitamin B12, either orally (through the mouth) in cases with insufficient dietary intake or intramuscularly (injected into the muscle) in cases of issues with absorption.
An excess of cobalt on the other hand, is generally not related to vitamin B12 levels, but rather levels of natural, elemental cobalt in the body. Unlike vitamin B12, this natural form of cobalt usually enters the body via environmental or occupational exposure through inhalation and contact with skin. However, in individuals that are not exposed occupationally, cobalt can enter the body with the use of implants made of cobalt, such as hip replacements. Both cases can result in cobalt toxicity which has accompanying effects, one of the most notable ones being cardiomyopathy–a condition which impairs the heart’s ability to pump blood. Research has made efforts to identify how cobalt implants in the body can contribute to cardiomyopathy; in particular, literature regarding cardiomyopathy has given attention to patients that have undergone cobalt hip implants. Given how rare such cases are, this topic remains an area of ongoing research.
Before further addressing the issue of cardiomyopathy as a result of cobalt hip implants, it would be helpful to understand the basics of hip replacements. The hip joint can be visualized as a “ball-and-socket” joint like pictured below. In this case, the femoral head acts as the ball, and the acetabulum serves as the socket. In hip implants, the femoral head (ball) can be replaced with metal or ceramic, and the acetabulum (socket) can be replaced with cobalt, polyethylene, or ceramic. If both the femoral head and acetabulum are metal, this is called a metal-on-metal or MoM joint; if the femoral head is metal but the acetabulum is polyethylene or ceramic, this is called a non metal-on-metal or non-MoM joint).
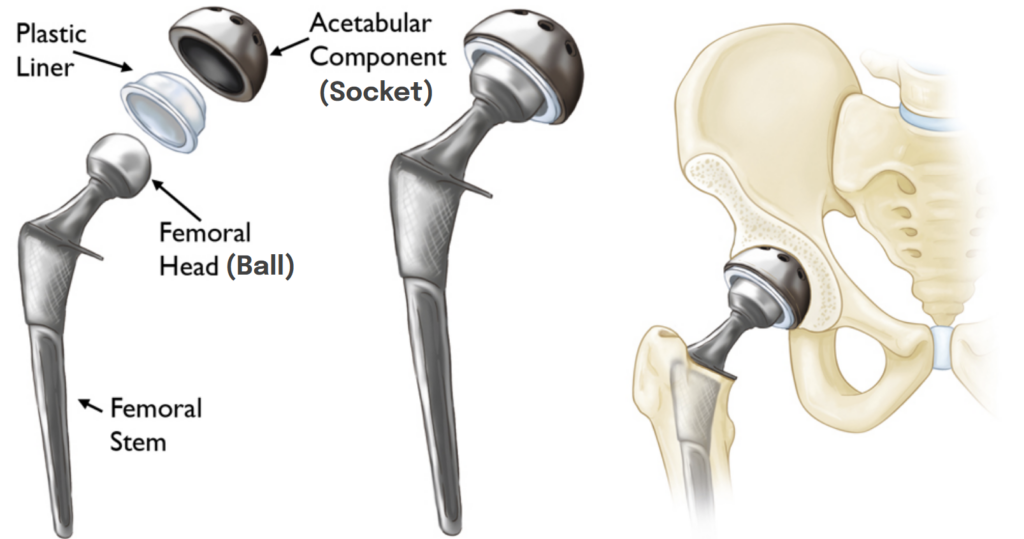
Cobalt, polyethylene, and ceramic are durable materials that are generally resistant to wear; however, wear can still occur. The issue arises when wear between these components causes cobalt particles to be released into adjacent tissues and the bloodstream, which can lead to toxic effects like cardiomyopathy. So, if a hip implant made from cobalt is found to be the cause of cardiomyopathy, how is it treated? First and foremost, the implant must be removed to eliminate the source of toxicity. In some cases, removal of the implant alone may be sufficient to treat cardiomyopathy. However, in more severe cases, removal of the implant may not be enough if the toxic effects of cobalt have progressed to a stage where a heart transplant is necessary due to heart failure, as you’ll see in some of the cases described further below.
The first review paper demonstrates the potential for patients with cobalt hip implants to experience cardiac damage even when levels of cobalt in the blood are relatively low. The researchers established a study group and a control group, both of which consisted of males over the age of 50. The study group consisted of 16 patients that had already undergone a MoM hip replacement surgery, and the control group consisted of 8 patients that had not undergone a hip replacement surgery, but were eventually scheduled to. The goal was to compare the cardiac function of both groups and identify key differences.
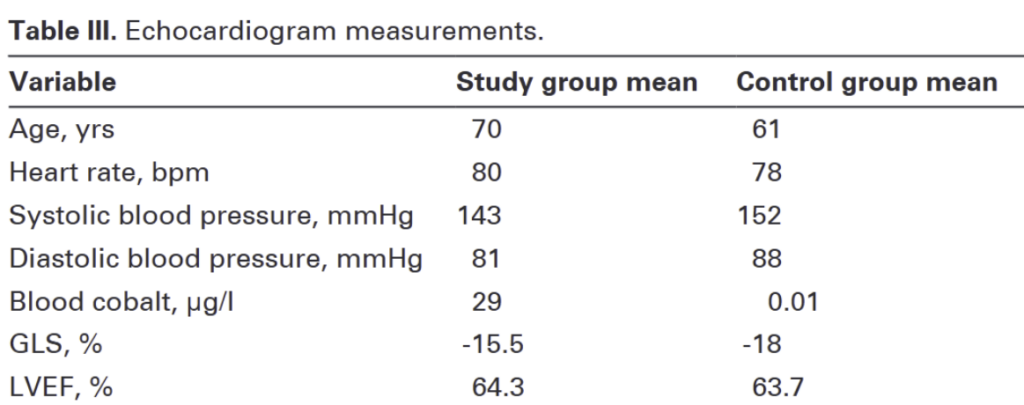
When assessing cardiac function, an echocardiogram is typically used; this is a tool that allows the heart to be visualized and determine cardiac function through various parameters. In this study, the researchers focused on 2 key parameters as a point of comparison: Left Ventricular Ejection Fraction (LVEF) and Global Longitudinal Strain (GLS). To break these terms down, we should first understand that the heart is composed of 4 chambers responsible for pumping blood; the main chamber which pumps blood to the rest of the body is called the left ventricle.
- LVEF measures how much blood is pumped out of its main chamber (left ventricle) with each heart beat. A healthy heart would pump out a greater percentage of blood (higher LVEF) than a dysfunctional heart (lower LVEF).
- GLS measures how much the heart’s muscle fibers shorten when the heart contracts. A healthy heart contracts more strongly, reflected by a higher (more negative) GLS value, whereas a weak heart contracts less strongly, reflected by a lower (less negative) GLS value GLS values are negative as it represents how much the heart muscle shortens when it contracts compared to when it’s relaxed–similar to how a sponge gets smaller when you squeeze it.
The key difference between these 2 metrics is that GLS is considered a more sensitive metric than LVEF in that it is able to determine changes in cardiac function in early stages of a disease that LVEF might miss. For example in cases of Heart Failure with Preserved Ejection Fraction (HFpEF), the heart is failing, but the LVEF reflects a normal value, telling an entirely different story. The researchers in this study highlight that GLS has not been used to assess cardiac function in patients with cobalt hip replacement prior, making it a compelling focus for this study.
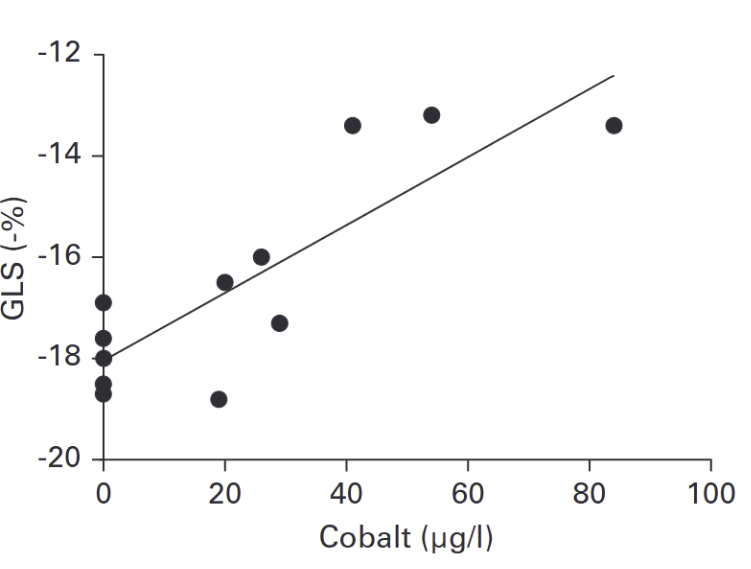
The researchers’ findings are noteworthy. Although the LVEF values of both the study and control group were nearly identical with no significant difference, GLS, revealed a significant distinction. The control group had a mean GLS of-18% (within normal range), whereas the study group had a mean GLS of -15.5% (within abnormal range associated with early heart failure). While the difference may not seem outstanding, it is a significant finding which highlights cardiac damage that LVEF alone failed to detect. Furthermore, the researchers found a strong correlation between cobalt levels in the blood and GLS, indicating that higher blood cobalt levels are associated with a lower GLS (weaker heart contraction). What we can take away from this is that patients with MoM hip implants have the potential to develop heart failure at early stages, and even with low amounts of cobalt in the blood, the risk of cardiac damage certainly exists. Looking at LVEF alone, one might assume that the MoM implants had no effect on the study group, which clearly wasn’t the case, as evidenced by the observed GLS.
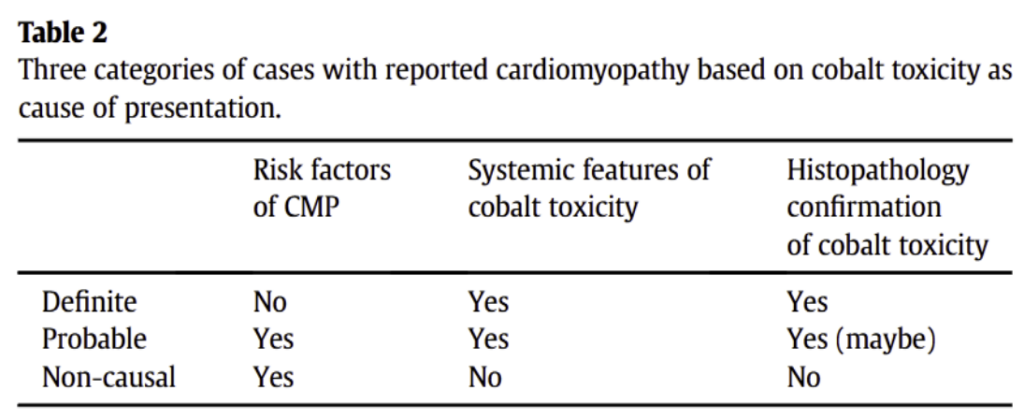
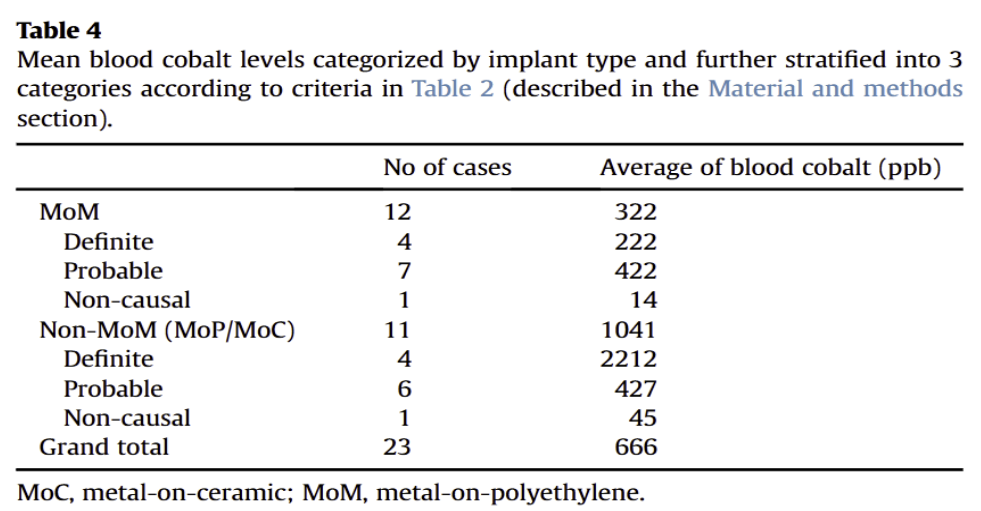
The second review paper analyzes cases from a database of patients that had undergone hip implants and subsequently developed cardiomyopathy. 23 cases were selected, 12 of them being patients with Non-MoM hip implants, and 11 of them being patients with MoM hip implants. The researchers wanted to determine whether the patients in this study experienced cardiomyopathy as a result of their hip implant, or due to other unrelated causes. They also compared cobalt levels of patients with MoM implants to patients with Non-MoM implants. Their methods involved stratifying the cases into three groups based on how likely it was that their cardiomyopathy was caused by their cobalt hip implant: definitely caused by the hip implant, probably caused by the hip implant, and not caused by the hip implant. They determined this likelihood based on whether patients already had risk factors of cardiomyopathy, whether patients displayed systemic effects of cardiomyopathy, and whether patients displayed histopathological confirmation of cobalt toxicity (confirmation of cobalt toxicity by examining body tissue under a microscope).
Interestingly, their analysis revealed that patients with MoM hip implants had a lower average level of cobalt in the blood compared to patients with Non-MoM hip implants. While one might assume that patients with MoM implants would have a higher average level of cobalt in the blood than patients with Non-MoM implants due to more cobalt being present in MoM implants, this finding could demonstrate that the type of implant is not the primary factor in determining such cases. What the researchers proposed as a possible explanation for this result was that the patients that had Non-MoM implants in this review had already undergone a hip replacement surgery once before; after getting a second revision surgery, there were components of the implant that remained from the first surgery which caused the new implant to wear and break down, resulting in a higher level of cobalt in the blood than patients with MoM implants, who did not have the same implant history prior.
Unique findings like the one in this study set the stage for specific case studies of cobalt related cardiomyopathy and its diverse clinical outcomes; following below is a brief overview of three cases of cardiomyopathy in patients that had undergone hip replacement surgery. Interestingly, in 2 of the 3 cases, a heart transplant was needed, whereas a heart transplant was not needed in 1 of the cases. This demonstrates the varying severity and progression of cobalt related cardiomyopathy.
Case 1: A 48 year old male developed cardiomyopathy symptoms after multiple hip replacement surgeries. The diagnosis was eventually narrowed down to cobalt toxicity by testing for cobalt levels in the blood. The metal hip implant was removed and cobalt levels in the blood decreased, but symptoms of heart failure were still present. Eventually, a heart transplant was decided and the patient returned to normal function following the transplant.
Case 2: A male in his early 30s developed cardiomyopathy symptoms after hip resurfacing surgery. The diagnosis was narrowed down to cobalt toxicity by testing for cobalt levels in the blood. The metal hip implant was removed and cobalt levels in the blood decreased, and cardiac function returned to baseline function. A heart transplant was not needed.
Case 3: A 52 year old male developed cardiomyopathy symptoms after multiple hip replacement surgeries. The diagnosis was narrowed down to cobalt toxicity by testing for cobalt levels in the blood. The metal hip implants were removed and cobalt levels in the blood decreased, but there was no improvement in heart function. Eventually, a heart transplant was decided and the patient had a great recovery following the transplant.
Ultimately, these cases and the supporting literature on the topic of cobalt related cardiomyopathy exemplify the need of a high index of suspicion to properly address such cases given its unpredictable and ambiguous nature. As proposed by researchers of this topic, long-term surveillance should be provided to patients that undergo procedures which use cobalt for implants to effectively prevent the development of cobalt toxicity and its accompanying effects like cardiomyopathy. In addition, given how rare such cases are, more research should be conducted to raise awareness and increase the knowledge surrounding cobalt related cardiomyopathy.
Sources:
Wolffenbuttel BHR, McCaddon A, Ahmadi KR, Green R. A Brief Overview of the Diagnosis and Treatment of Cobalamin (B12) Deficiency. Food and Nutrition Bulletin. 2024;45(1_suppl):S40-S49. doi:10.1177/03795721241229500
Jenkinson MRJ, Meek RMD, Tate R, MacMillan S, Grant MH, Currie S. Cobalt-induced cardiomyopathy – do circulating cobalt levels matter?. Bone Joint Res. 2021;10(6):340-347. doi:10.1302/2046-3758.106.BJR-2020-0414.R2
Mark, Dominic, Tate R, et al. Cardiac function may be compromised in patients with elevated blood cobalt levels secondary to metal-on-metal hip implants. The Bone & Joint Journal. 2024;106-B(3 Supple A):51-58. doi:https://doi.org/10.1302/0301-620x.106b3.bjj-2023-0814.r1
Umar M, Jahangir N, Faisal Khan M, Saeed Z, Sultan F, Sultan A. Cobalt cardiomyopathy in hip arthroplasty. Arthroplasty Today. 2019;5(3):371-375. doi:https://doi.org/10.1016/j.artd.2019.04.010
Castrillo Bustamante C, Canteli Álvarez Á, Burgos Palacios V, et al. A case report of cobalt cardiomyopathy leading to electric storm and cardiogenic shock: the importance of the orthopaedic background in patients with heart failure of unknown aetiology. Eur Heart J Case Rep. 2021;5(4):ytab057. Published 2021 Apr 5. doi:10.1093/ehjcr/ytab057
Rahman TM, Hall DJ, Darrith B, et al. BMJ Case Rep 2022;15:e249070. doi:10.1136/bcr-2022-249070
Szedlak P, Virdi A, Cacciottolo P, Shepherd S, Pettit S, Falter F. Cardiac Transplantation following Cobalt Cardiomyopathy from Bilateral Metal-on-Metal Hip Replacements. Case Rep Anesthesiol. 2022;2022:3373363. Published 2022 Jun 8. doi:10.1155/2022/3373363


