It has long been known that over-exposure to copper leads to cell death. Until recently, however, the exact mechanism behind copper-induced cell death was unknown and assumed to be due to the accumulation of Reactive Oxygen Species (ROS), which unbound copper produces, and causes damage to DNA among many important molecules in the cell. This cellular damage is recognized by the cell and results in apoptosis, a form of programmed cell death that causes the cell to sacrifice itself for the good of the organism. It was assumed that copper-induced cell death was caused by apoptosis, as opposed to several other programmed cell death pathways. In 2022, Peter Tsvetkov published “Copper induces cell death by targeting lipoylated TCA cycle proteins,” showing a) experimental evidence that copper-induced cell death is a unique class of programmed cell death dubbed “cuproptosis,” and b) several hallmark molecular features define cuproptosis and set it apart from other cell death pathways.
First, they needed to show that cuproptosis is a programmed cell death, as opposed to cell death caused directly by copper toxicity. A key feature of programmed cell death pathways is the amount of time it takes for cell death to occur after exposure to the death-causing stimulus, taking 12 hours to multiple days to occur. To test this, the researchers exposed cells to copper for two hours, then rinsed the cells of copper so that any effects would only be due to the initial exposure. By measuring how many cells were still alive over the following 96 hours, the researchers showed that the copper exposure resulted in delayed cell death – strong evidence that cuproptosis is programmed.
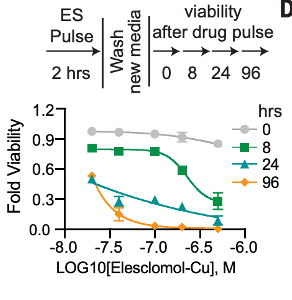
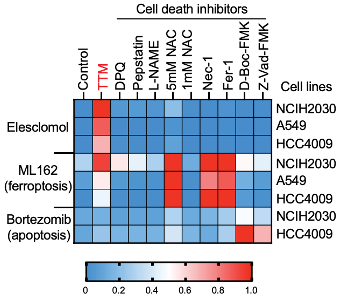
To determine if cuproptosis is distinct from other programmed cell death pathways like apoptosis, the researchers used several cell death inhibitors known to stop apoptosis, ferroptosis, and others. They saw that only a copper chelator, which binds copper and reduces the effective copper concentration in the cell, was able to stop cuproptosis, while the other cell death inhibitors had no effect. This showed that cuproptosis is, in fact, a different pathway from other known programmed cell deaths.
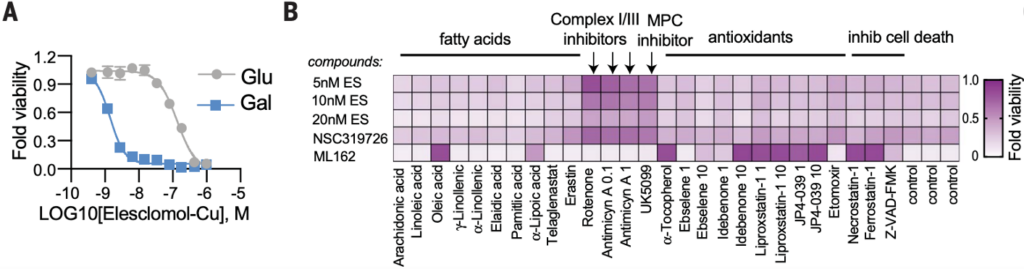
While these findings distinguished cuproptosis as a distinct form of programmed cell death, it was still unclear what the pathway is and how copper is triggering it. Because copper is vital to several steps in the production of ATP – cellular energy – in the mitochondria, they did a series of experiments to determine if cuproptosis relied on any of those steps. First, they tested whether supplementation of cells with glucose, a sugar which cells can make ATP from without many mitochondrial functions, rescued cells from cuproptosis compared to cells in galactose, a sugar that requires oxygen and the Kreb’s (TCA) cycle to produce ATP. They found that glucose did rescue cells from cuproptosis, meaning that reliance on oxygen and the TCA cycle in mitochondria was required for cuproptosis. Next, they did a screen to see if the addition of various metabolites used in cellular respiration, as well as cell respiration inhibitors, had an effect on cuproptosis. They found that the cell respiration inhibitors, and importantly inhibitors of the mitochondrial pyruvate complex (MPC), rescued cells from cuproptosis, further implicating and specifying the necessity of the TCA cycle for cuproptosis.
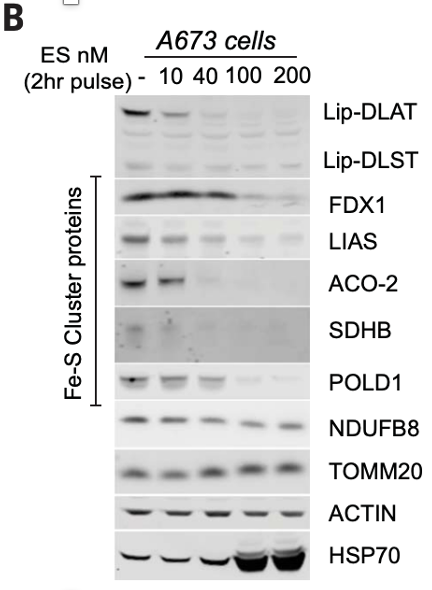
With their focus on the TCA cycle, they were able to do genetic knockout screens to test if various genes related to the TCA cycle were required for cuproptosis. They identified 7 genes, four involved protein lipoylation, a post-transcriptional protein modification, and three (of four total) protein targets of lipoylation. After determining that copper has a high affinity for lipoic acid and lipoylated protein, they used western blotting, a protein visualization assay, to identify three hallmark features of cuproptosis. First, the TCA cycle of cuproptotic cells is disrupted by copper-induced agglomeration of lipoylated protein (oligomerization). Second, cuproptotic cells lose iron-sulfur cluster proteins which are vital for cell respiration and DNA repair, among other vital cell processes. Finally, cuproptoptotic cells have an increased amount of the protein HSP70, which mediates proper protein folding and is upregulated when proteins are misfolding or damaged (proteotoxic stress). These features can be seen in the western blot below of protein cells undergoing cuproptosis, with copper concentration increasing from left to right. Lipoylated protein oligomerization can be seen in the numerous faint bands labeled Lip-DLAT, decreasing in intensity but increasing in number from left to right. Loss of Fe-S cluster proteins is seen in the labeled bands which decrease as copper concentration increases. Lastly, the bottom band shows HSP70 presence increasing with copper concentration.
The findings of Tsvetkov’s paper have been used in countless ways to investigate possible therapeutic strategies for various diseases – the paper has been cited over 600 times since its publication two years ago. One of these papers is “Copper Deposition in Polydopamine Nanostructure to Promote Cuproptosis by Catalytically Inhibiting Copper Exporters of Tumor Cells for Cancer Immunotherapy,” published by Yongyong Li in 2024. In the paper, Li and his team use a nanoparticle, Polydopamine, to deliver large amounts of copper to breast cancer tissue to stop tumor growth and elicit an immune response to further fight the tumor. After first seeing positive results in the in vitro treatment of tumor tissue with the copper nanoparticles, seeing cell death, indicators of programmed cell death, and a distinct immune response, they tested the treatment on mice with the same type of tumor tissue inserted in their flank. They saw that the copper nanoparticles were able to accumulate and stay in the tumor, increasing copper concentration in the tumor, and significantly reducing tumor growth compared to control treatments.
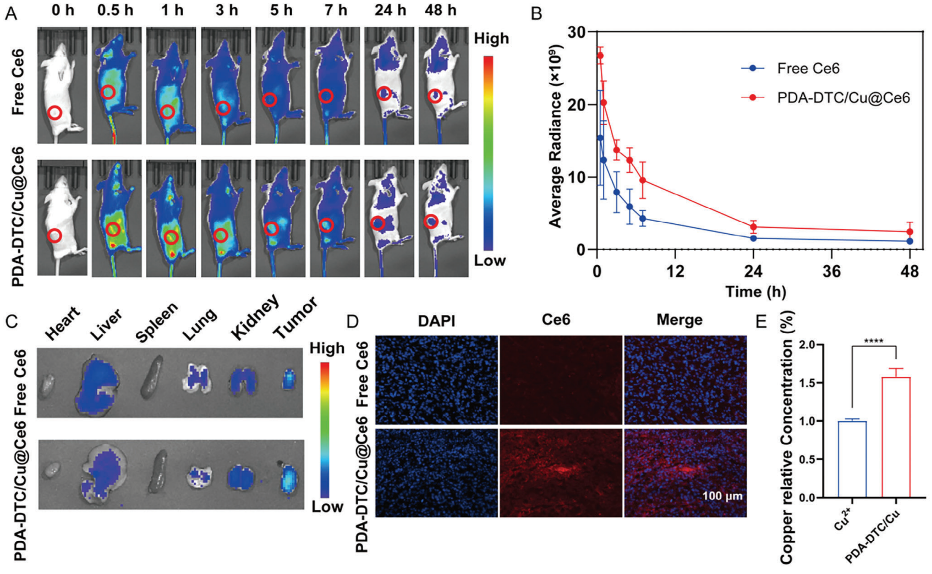
Similarly, they saw a distinct increase in numerous anti-tumor immune responses in mice treated with the copper nanoparticles compared to controls.
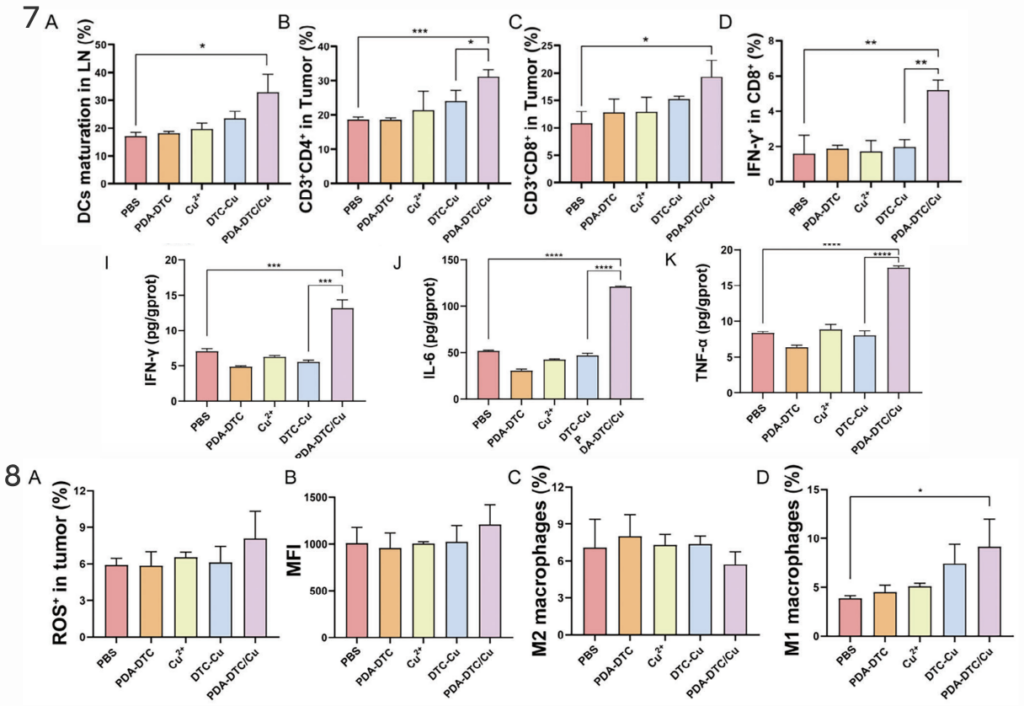
Given the success of the copper nanoparticle treatment in reducing tumor growth and producing anti-tumor immune responses, it will be exciting to see expanded tests and trials of the treatment in subsequent studies.
Cuproplasia is the inverse of cuproptosis, an umbrella term encompassing various cellular mechanisms relying on copper for the production of new cells (proliferation). Christopher Chang and his lab summarize emerging cell pathways of cuproplasia, especially as they relate to cancer in their 2020 review paper, “Connecting copper and cancer: from transition metal signaling to metalloplasia.” The review also discusses cuproptosis, however, the subsequent publishing of Peter Tsvetkov’s foundational paper described above has rendered most of their discussion outdated. In discussing cuproplasia, the authors mainly focus on the use of copper by protein kinases (PKs) to elicit cell proliferation signaling cascades. PKs are enzymes that phosphorylate (attach a phosphate group to) other proteins – which in turn alters the function of the targeted function. The copper-dependent activity of PKs like MEK1/2 is vital in cell signaling cascades which result in cell proliferation. ULK1/2, on the other hand, uses copper to produce a cell signaling cascade that digests proteins for the recycling of their component amino acids (autophagy), a process necessary in tumors, where cells are growing and dividing faster than nutrients like amino acids can be adequately delivered
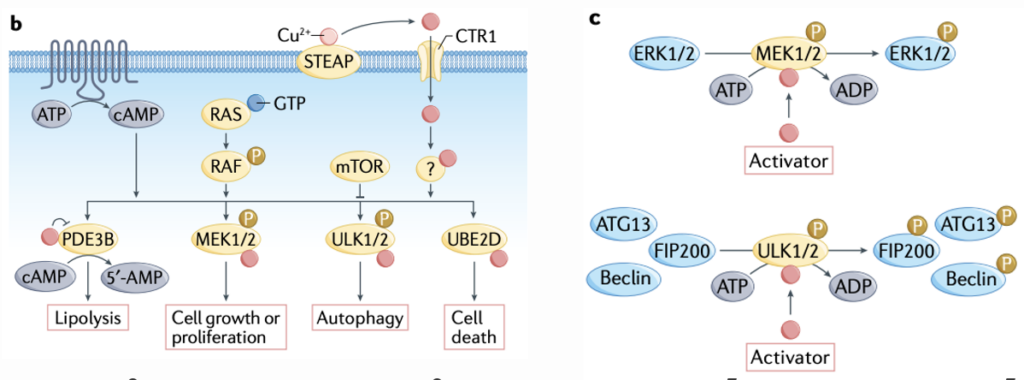
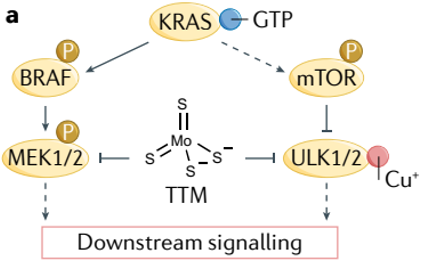
Contrasting the cancer therapeutic approach utilizing cuproptosis, therapies targetting cuproplasia typically use copper chelators, which bind and reduce the effective copper concentration in the cell. The authors discuss several studies effectively using copper chelators to stop tumor growth. One used TTM, the copper chelator used by Tsvetkov to rescue cells from cuproptosis, to stop breast cancer growth by blocking the Braff-driven oncogenic pathway, which is mediated by MEK1/2 as described above.
One interesting application of characterized cuproplasia pathways is demonstrated in the 2023 paper, “Cuproplasia characterization in colon cancer assists to predict prognosis and immunotherapeutic response,” by Binbin Cui. In the paper, the authors use a number of gene set analysis methods to generate a novel set of 6 genes that are correlated to a set of cuproplasia-related genes in colon cancer samples. Interestingly, most of the 6 identified genes have been studied very little and were previously unidentified in relation to cuproplasia. The authors used these six genes to generate a Cuproplasia risk Score (Cu_riskScore) which successfully predicted patient survival outcomes across a large set of colon cancer patients. Going further, they were able to correlate Cu_riskScore to tumor response to immunotherapy, opening up the possibility of using Cu_riskScore to determine if immunotherapy is the right option for individual patients with colon cancer. The paper presents evidence of correlation outside of the data set used to produce the Cu_riskScore algorithm in the first place, and more analysis is needed to determine if Cu_riskScore is a viable risk stratification method across broader populations.
In summary, cuproptosis and cuproplasia represent inverse functions of copper in the cell, copper-induced programmed cell death and copper-mediated cell proliferation, respectively. The two processes point to distinct possibilities for improvement in cancer treatment through the development of novel treatment strategies, as in the development of copper-coated nanoparticles by Li, et al., and of new methods of risk stratification for patients to determine the viability of minimally invasive, existing therapeutic approaches, as in the generation of Cu_riskScore by Cui, et al..
References:
- Ge, E.J., Bush, A.I., Casini, A. et al. Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat Rev Cancer 22, 102–113 (2022). https://doi.org/10.1038/s41568-021-00417-2
- J. Chang, W. Yin, H. Zhi, S. Chen, J. Sun, Y. Zhao, L. Huang, L. Xue, X. Zhang, T. Zhang, H. Dong, Y. Li, Copper Deposition in Polydopamine Nanostructure to Promote Cuproptosis by Catalytically Inhibiting Copper Exporters of Tumor Cells for Cancer Immunotherapy. Small 2024, 20, 2308565. https://doi.org/10.1002/smll.202308565
- Peter Tsvetkov et al. ,Copper induces cell death by targeting lipoylated TCA cycle proteins.Science375,1254-1261(2022).DOI:10.1126/science.abf0529
- Zhang, Bomiao, et al. “Cuproplasia characterization in colon cancer assists to predict prognosis and immunotherapeutic response.” Frontiers in Oncology, vol. 13, 16 Mar. 2023, https://doi.org/10.3389/fonc.2023.1061084.


