Zinc is an important nutrient, which plays an important role in the function of over 300 enzymes and transcription factors, which are essential for almost every cell in our body. It’s especially important for our immune system, helping it develop and function properly. When we do not get enough zinc, it can lead to a variety of diseases, like stunted growth, problems with the thymus (which is key for immune function), and a weakened ability to fight infections. You might also notice slower healing from injuries. These problems are due to zinc’s influence on cell growth, differentiation, and the delicate balance of signaling pathways.
To keep our cells healthy, it is crucial to regulate zinc levels in our bodies. This is where zinc transporters come in. They help manage the flow of zinc in and out of cells, ensuring we have just the right amount. There are two main types: ZnT (Zinc Transporters) and ZIP (Zinc Importers) (Fig 1)
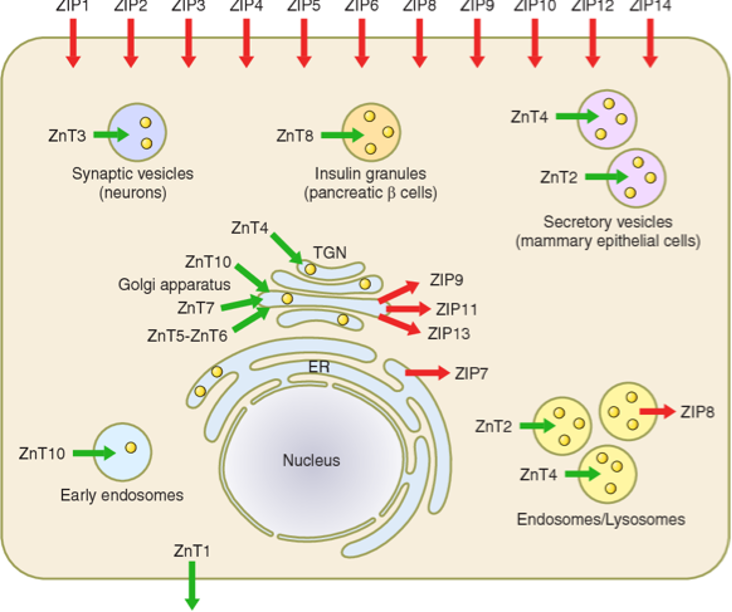
Fig 1. The subcellular localization of ZIP and ZnT transporters
Zinc Transporters (ZnT): ZnT transporters help keep zinc levels balanced within cells, making sure there is enough for essential functions while preventing toxicity into the cell. Different ZnT transporters are found in various tissues, each with specific roles. The key transporters are:
- ZnT1: Mainly exports zinc out of cells to prevent toxicity.
- ZnT2: Brings zinc into specific compartments within cells, especially in mammary glands and the prostate.
- ZnT3: Helps regulate zinc levels in neurons, which is crucial for brain function.
- ZnT8: Plays a role in insulin regulation.
When these transporters do not function properly, it can lead to health issues, including developmental problems, immune disorders, and even neurodegenerative diseases.
ZIP Transporters: On the other side, ZIP transporters help cells absorb zinc from their surroundings, increasing intracellular zinc levels. This is important for maintaining overall zinc balance in the body. Adequate zinc is essential for cell growth and signaling, which are vital for overall health.
Zinc’s role in cell growth is well recognized. The cell cycle in eukaryotic cells includes several phases: the G1 phase (gap 1), S phase (DNA synthesis), G2 phase (gap 2), and M phase (mitosis). Each phase is tightly regulated to ensure everything is completed before moving on to the next step. (Fig 2)
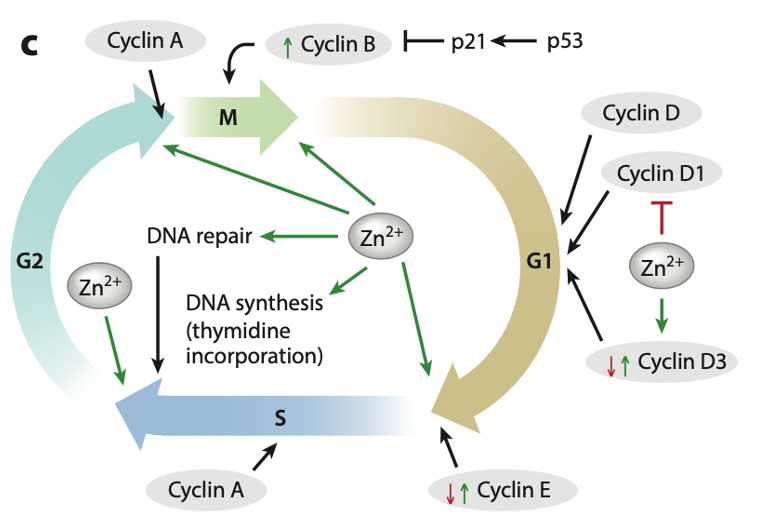
Figure 2. Zinc impact on cell cycle
One of the key players in this process is p53, a protein that helps regulate the cell cycle and apoptosis (the process of programmed cell death). Interestingly, while zinc does not directly affect how much p53 is made, a lack of zinc can make p53 less stable and less effective.
Zinc also plays a crucial role in various metabolic pathways, such as the insulin signaling pathway, which helps store and transport glucose in pancreatic beta cells. When zinc levels are decreased, it can affect this pathway, potentially leading to conditions like diabetes.
In the first article titled “Zinc Deficiency Affects Insulin Secretion and Alters Insulin-Regulated Metabolic Signaling in Rats,” the authors investigated how different zinc levels impact pancreatic beta cells and insulin secretion. They also examined how zinc deficiency affects glucose intolerance and insulin function in key organs. To do this, they proposed two trials with rats divided into three groups: one group received a standard diet (ZnC), another was fed a zinc-free diet (ZnF), and the last group started on a zinc-free diet before switching back to a standard diet (ZnFC).
To analize the hypothesis, they conducted mRNA expression analysis using quantitative RT-PCR (qPCR) and extracted total RNA from liver and white adipose tissue samples.
They also performed immunostaining to detect specific proteins in tissues or cells, which can be observed under a microscope or using fluorescence. They used various markers to identify immune cells, such as CD11b/c for dendritic cells and CD68 for total macrophages. To check for fibrosis, they looked at collagen I and α-SMA, and they used insulin to identify pancreatic beta cells.
Additionally, they took pancreatic sections to detect M1 and M2 macrophages using CD169 and CD163 markers, respectively. They also performed oral glucose tolerance tests and intraperitoneal insulin tolerance tests, measuring plasma concentrations to calculate the copper/zinc (Cu/Zn) ratio, since copper levels are influenced by zinc availability.
The results revealed that dietary zinc levels significantly affect insulin secretion and the function of insulin-producing organs. Zinc deficiency led to lower insulin production, which negatively impacted glucose and lipid metabolism in the liver and fat tissues.
The higher Cu/Zn ratio in the zinc-deficient group indicated increased copper absorption due to low zinc levels, proposing possible inflammatory processes. Moreover, insulin target gene expression was adversely affected in zinc-deficient rats, showing the problem of insulin deficiency. Despite lower insulin levels, both fat breakdown (lipolysis) and fat creation (lipogenesis) were elevated, suggesting an imbalance in lipid metabolism. Increased inflammation markers, particularly M1 macrophages in the pancreas, pointed to a pro-inflammatory environment contributing to beta-cell dysfunction.
The study concluded that zinc deficiency hampers insulin secretion by triggering beta-cell failure, affecting metabolic signaling in the liver and fat tissues.
In the following article titled “Zinc Deficiency Promotes Testicular Cell Apoptosis in Mice,” researchers established that zinc is crucial for sperm production. They also found that carbon tetrachloride (CCl₄) induces oxidative damage and cell death in the testes. This study aimed to explore how zinc deficiency, when combined with CCl₄ treatment, impacts testicular apoptosis and the mechanisms involved.
It is essential to consider the factors contributing to cell death and oxidative damage. One major factor is reactive oxygen species (ROS), which are highly reactive oxygen-containing molecules produced during metabolic processes, especially during mitochondrial respiration and the citric acid cycle. When ROS accumulate, they can lead to oxidative stress. Normally, antioxidants like glutathione (GSH) and superoxide dismutase (SOD) help manage ROS levels, maintaining a balance. But too much ROS can cause damage to cellular lipids, proteins, and nucleic acids.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is crucial for antioxidant defense, regulating various antioxidant molecules to protect against oxidative stress. CCl₄ is a well-known hepatotoxic agent that induces significant oxidative stress and can lead to various health problems.
Apoptosis, the orderly death of cells, is vital for maintaining balance in the body. Normally, pro-survival factors like Bcl-2 work against pro-apoptotic factors like Bax. When this balance is disrupted, like in cases of zinc deficiency or oxidative stress from CCl₄ germ cell apoptosis can occur. The protein p53, as mentioned earlier, is key in apoptosis and spermatogenesis. It promotes cell death in response to oxidative stress by activating Bax and inhibiting Bcl-2, leading to the release of cytochrome c and triggering a mitochondrial-dependent cell death pathway. Additionally, caspase-3 is crucial for apoptosis, helping to initiate the process by cleaving poly (ADP-ribose) polymerase (PARP).
In this study, researchers aim to explore the effects of zinc deficiency on male mice. They divided the mice into two groups: one group received enough zinc, while the other was put on a low-zinc diet for a certain period. The control group stayed on a regular diet.
To confirm zinc deficiency, they measured zinc levels in the blood and tissues using atomic absorption spectrometry. They also checked ROS levels in testicular tissues using various assays, like fluorescence and spectrophotometry. For assessing apoptosis, they employed:
- TUNEL Assay: This helps spot broken DNA, a sign of cell death.
- Histological Examination: This involved looking at the tissue under a microscope to detect changes related to apoptosis.
The findings emphasized the importance of zinc for male reproductive health, as it is found in higher concentrations in testicular tissue crucial for sperm production. When zinc levels drop, male fertility can suffer, leading to a loss of germ cells and lower sperm quality.
The combination of zinc deficiency and exposure to CCl₄ leads to oxidative stress, with higher ROS levels and lower antioxidants like SOD and GSH. Zinc deficiency also activates p53, contributing to mitochondrial apoptosis. On the other side, getting enough zinc can help reduce oxidative damage and boost antioxidant levels.
Overall, these studies highlight just how vital zinc is and stress the importance of maintaining healthy zinc levels throughout our lives.
Sources:
Chen Y, Yang J, Wang Y, Yang M, Guo M. Zinc Deficiency Promotes Testicular Cell Apoptosis in Mice. Biol Trace Elem Res. 2020 May;195(1):142-149. doi: 10.1007/s12011-019-01821-4. Epub 2019 Jul 16. PMID: 31309446.
Nakamura, A., Kido, T., Seki, Y., & Suka, M. (2024). Zinc deficiency affects insulin secretion and alters insulin-regulated metabolic signaling in rats. Journal of Trace Elements in Medicine and Biology, 83, 127375. https://doi.org/10.1016/j.jtemb.2023.127375
Wessels I, Fischer HJ, Rink L. Dietary and Physiological Effects of Zinc on the Immune System. Annu Rev Nutr. 2021 Oct 11;41:133-175. doi: 10.1146/annurev-nutr-122019-120635. Epub 2021 Jul 13. PMID: 34255547.


