Iron is an important micronutrient that is involved in many important processes in the body, one of these important processes is the ability of the iron to transport oxygen through haemoglobin in the red blood cells. If there is a reduction in oxygen carrying capacity it can lead to condition known as anaemias. Recently scientists have investigated a new molecule known as Fibrinogen like protein 1 or FGL1 which is a protein made in the liver. Two new studies explore the effects that FGL1 have in iron metabolism particularly iron homeostasis and cell death caused by iron through a process known as ferroptosis.
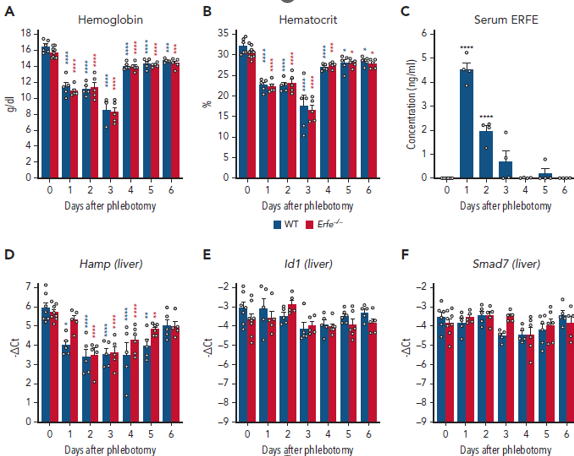
The first research identifies the role of FGL1 in recovering of anaemia. Anaemia as already mentioned is a disease that is characterised by decreased oxygen carrying capacity in the blood. There are various reasons for anaemias but the largest causes in the past few years were malaria, thalassemia, dietary iron deficiency and sickle cell anaemia. Scientists from France came up with the theory that after haemorrhage there is recovery from anaemia in mice although previously identified regulator Erythroferrone (ERFE) that suppresses Hepcidin which is the master regulator of iron in the body is not enough to overcome the anaemia induced by haemorrhage. They removed the gene from mice that was encoding ERFE and checked whether after draining some blood from these mice helps them to recover from anaemia as compared to the normal mice. And to their surprise their hypothesis was correct, the mice without the ERFE gene were recovering from anaemia proving there is another factor that regulates Hepcidin suppression. They also proved that FGL1 was able to suppress hepcidin in experiments done in the lab and on rats that were anaemic. Along with this they were able to show which part of FGL1 is causing this suppression of Hepcidin as by removing FGL1 gene from mice causes an increase in Hepcidin expression. Overall, this research opened new questions related to haematology (study of blood) by exploring the unknown functions of FGL1. The results below prove this scientific discussion.
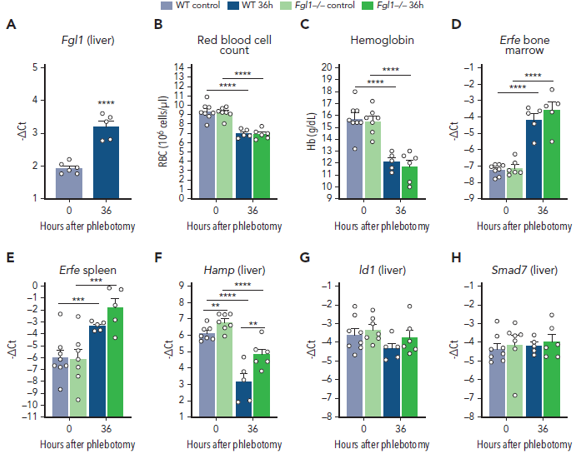
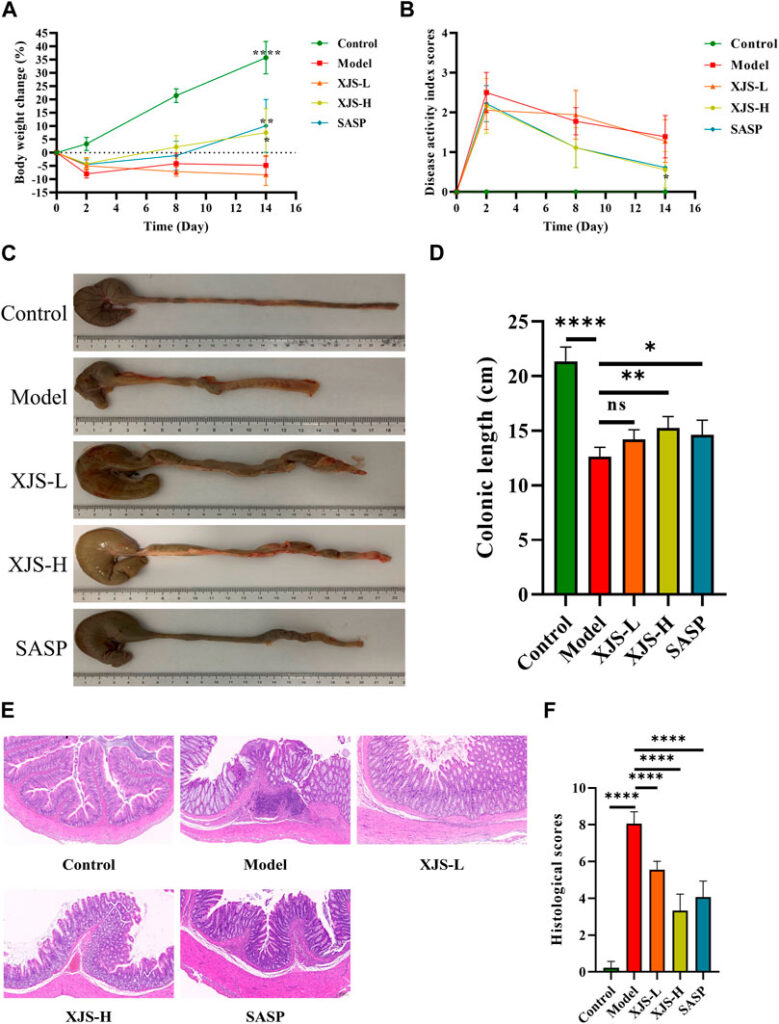
Another study explores the effect of a Chinese herbal medicine known as Xie Jue San (XJS) which is a mixture of two plant extracts known as Dragons Blood and Myrrha on targeting FGL1 which is implicated as a potential therapeutic (through a previous study done by these scientists) in causing the inflammatory bowel disease known as Crohn’s Disease. The scientists through modern technological analysis proved in another study done in their lab previously that XJS might be able to alleviate inflammation in Crohn’s disease. The main mechanism that infuriates this disease is ferroptosis, which is a type of cell death that is caused due to iron overload and stress like environment in the cell. Ferroptosis is caused due to FGL1 and other inflammatory mediators that augment the effect of each other. The scientists performed experiments on rats that were mimicking colitis and treated them with XJS. They were able to find out that the XJS treated rats were showing better signs to alleviate inflammation through macroscopic and microscopic examinations as compared to the rats that were not treated with XJS. They also proved that the inflammation causing chemicals were reduced in the colon tissues of the rats treated with XJS as compared to the disease group. Along with this they checked the balance of harmful damaging molecules to non harmful oxidants and they found that XJS treated groups had more non harmful oxidants. They also found that the gene expression of the pathway that made more FGL1 and eventually triggered this disease was downregulated in the XJS treated group. In conclusion XJS might be considered as a therapy for Crohn’s disease as it is able to ameliorate the disease by repressing Ferroptosis through the inhibition of the inflammatory pathway involved in the synthesis of FGL1. The results below prove this scientific discussion.
Sources:
Sardo, Ugo, et al. “The hepatokine FGL1 regulates hepcidin and iron metabolism during anemia in mice by antagonizing BMP signaling.” Blood 143.13 (2024): 1282-1292.
Gao, Ying, et al. “Xue-Jie-San restricts ferroptosis in Crohn’s disease via inhibiting FGL1/NF-κB/STAT3 positive feedback loop.” Frontiers in Pharmacology 14 (2023): 1148770.


