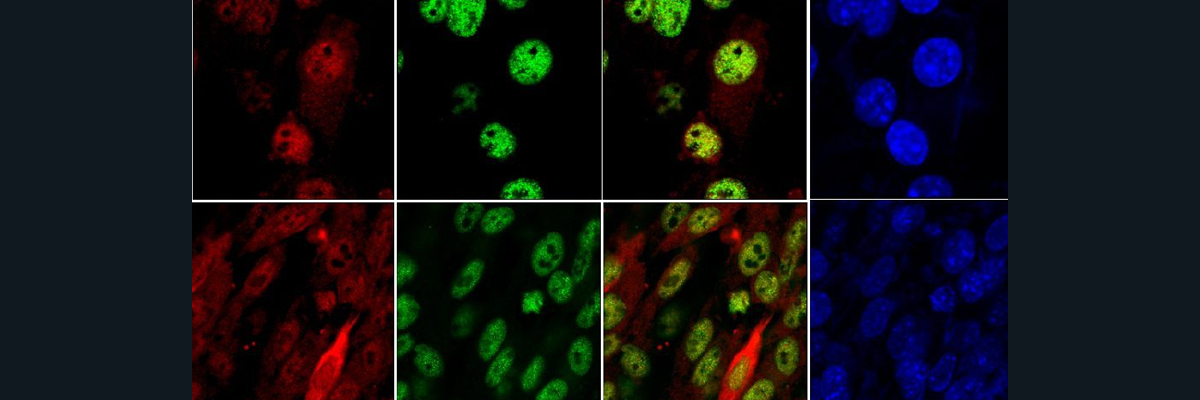
Epidermodysplasia verruciformis (EV) is a disease characterized by being more susceptible to β-Human Papillomaviruses (HPV), which is a strain of HPV. The primary clinical symptom is the formation of tons of wart-like lesions on patients. In one case, a man with EV had an operation to undergo the removal of 12 pounds (~5.5 kilos) of warts. Unfortunately, in many cases, when the lesions of people with EV are exposed to the sun, they become skin cancer. Since there are no well-known treatments other than preventing sun exposure, and surgery, if the lesions become cancerous the only option is surgical intervention. EV patients are otherwise healthy; they only are more susceptible to β-HPV, not to any other illness or disease. It should also be noted that in the paper cited, all the patients evaluated were the product of incestuous unions. There are two types of EV patients, ones that are typical, meaning they have protein deficiencies from birth that cause the disorder, and atypical EV patients, whose inability to fight off β-HPV comes from being immunocompromised. The incidence rate iof EV is ~1.43×10-15%, making it extremely uncommon.
Traditionally, it was thought that zinc (Zn) imbalance played a role in the disease, particularly because the two proteins, EVER1 and EVER2 (encoded for by the TMC6 and TMC8 genes, respectively), that most EV patients are deficient or defective with interact with a protein, ZnT1, that is responsible for moving Zn out of the cell. When there is more EVER1 or EVER2 in a cell than there should be, Zn expulsion from the cytoplasm of a cell increases, so researchers thought that EV relied on imbalance of Zn in the cell because most EV patients are deficient in EVER1 or EVER2. However, the researchers in this paper sought to discover what genetic deficiency was causing the disease in their patients. In that vein, they conducted a genome wide survey of their patients and the patients’ families looking for areas on chromosomes that were particularly associated with the expression of EV. They settled on a given area of the fifteenth chromosome that all the patients had in common, and which all had a rare mutation that caused a deleterious effect in a protein called CIB1 (Fig1. A,B,D). EVER1 and EVER2 were not implicated in these patients’ manifestations of EV as the genes that encode them were outside of the area of interest that the researchers identified, however, the two EVs are clinically indistinguishable from one another (i.e. they have the exact same symptoms). Further, since β-HPV has a unique presentation in that it only affects the skin, it was postulated that in healthy people CIB1 must be abundant in the dermis and in hair follicles. CIB1 was found to be abundant in these areas in healthy people, but not in patients with EV. (Fig1. C,D).
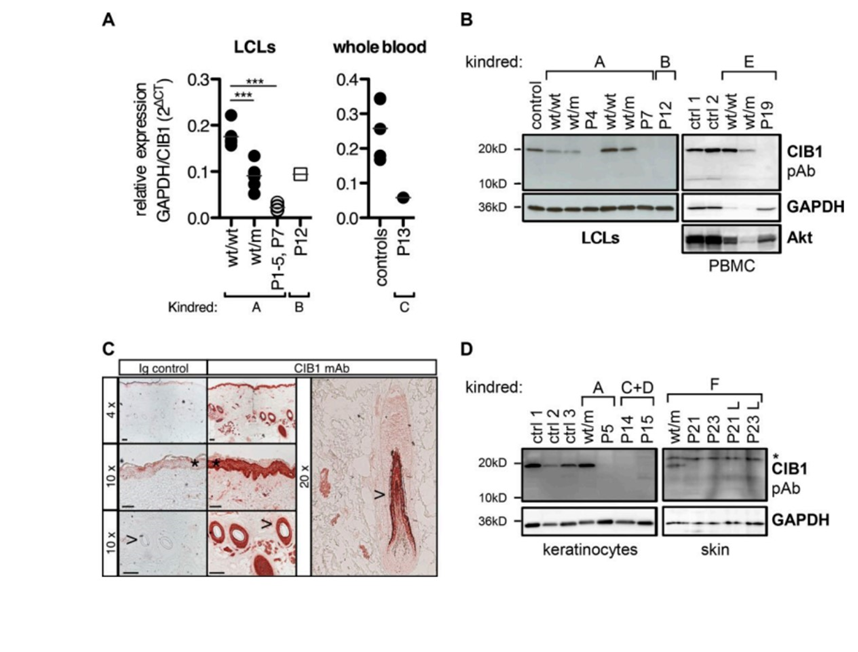
Figure 1: A demonstrates differences in CIB1 protein in Lymphoblastoid cell lines and whole blood in patients. B indicates the lack of CIB1 expression in homozygous recessive patients. C expresses the abundance of CIB1 in skin and hair follicles. D exhibits how CIB1 is not expressed in the skin and hair follicles of patients with EV.
Additionally, CIB1’s presence in the cell is dependent on the presence of EVER1 and EVER2 (Fig2. C,D,E). The researchers determined that when a cell is deficient in either EVER1 or EVER2 it is also deficient in CIB1, but inducing EVER1 or EVER2 into cells that have the gene for CIB1 and are deficient in either EVER protein returns the expression of CIB1, suggesting that the EVER proteins are necessary to CIB1 expression (Fig2. C,D). Moreover, when CIB1 is expressed by itself, its location is in the nucleus, while when CIB1 is expressed with the EVER proteins, it moves to the region where the EVER proteins are found, typically the perinuclear and cytoplasmic areas, indicating that they form a complex together (Fig2. F).
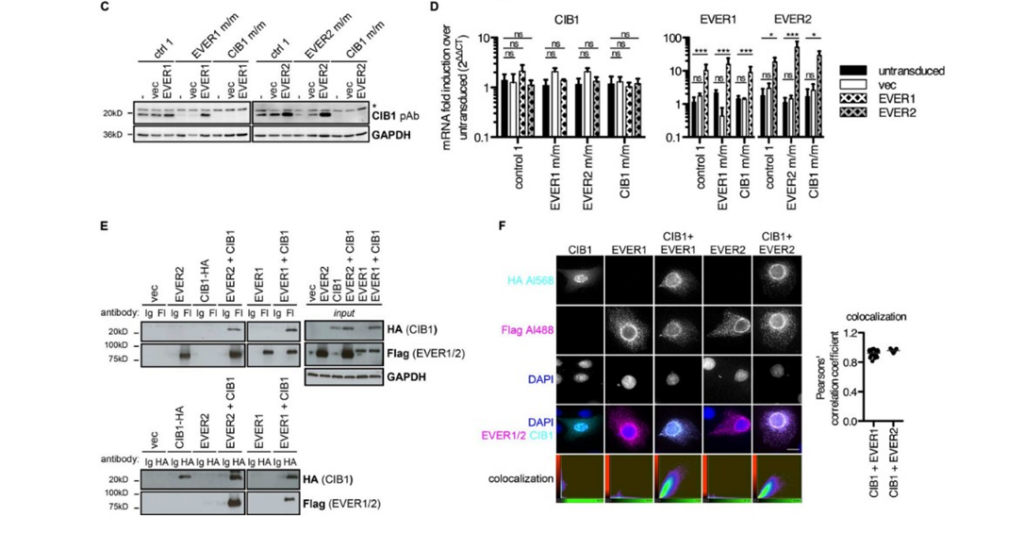
Figure 2: C demonstrates that returning EVER expression to the cell when the patient can produce CIB1 returns CIB1 expression, but the reverse is untrue, that is CIB1 expression in people who are unable to produce CIB1 does not return the expression of the EVER proteins. D demonstrates that when the same procedure as in C is undertaken, there is no difference in CIB1 mRNA levels. E expresses how the EVER proteins are expressed with CIB1. F explains the localization of CIB1, first by itself, and then when expressed with the EVERs how they colocalize.
Additionally, the researchers determined that the level of Zn within the cell is unchanged regardless of whether EVER1, EVER2, or CIB1 is expressed in excess, indicating that Zn does not play a role in EV (Fig3. A,B,C). Moreover, in order to explain how people with EV are healthy other than their susceptibility to β-HPV, the researchers investigated whether EVER1, EVER2, or CIB1 affected the normal immune response by looking at one of the most well-known immune signals and one of the most famous cells involved in immunity (TNF-α and NFκ-β cells respectively) in patients that were deficient in these proteins (Fig3. D,E). These patients demonstrated no difference between a healthy person and themselves in these categories (Fig3. D,E).
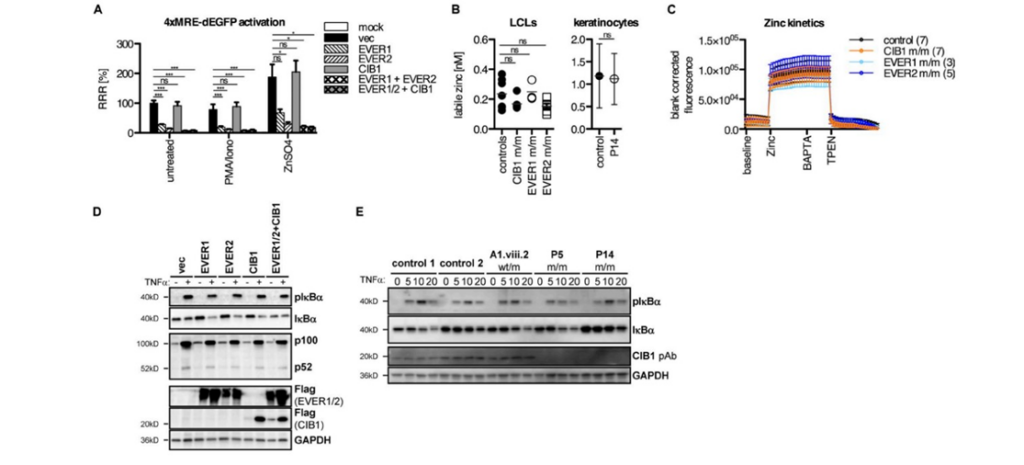
Figure 3: A denotes no difference between Zn levels when CIB1 is present in excess quantities, while it does emphasize the impact EVER1 and EVER2 have on Zn levels when they are in excess, while have CIB1 in excess with them has no impact on intracellular zinc. B expresses zinc levels in control patients and CIB1 and EVER deficient people in both lymphoblastoid cell lines and hair follicles are relatively similar. C demonstrates that zinc kinetics within the cell were within normal range for every individual tested. D and E express that there is no difference in basic immune response between EV patients and healthy people.
Moreover, researchers hypothesized that EV only manifests itself when the patient is exposed to β-HPV. To test this, the researchers looked at home long it would take a wound in a line of cells to heal (something that CIB1 plays a role in). In CIB1 deficient people, however, there was no difference between them and the healthy patient in terms of how long it took for the cells to heal the wound, indicating that the expression of EV and CIB1 deficiency only manifests when exposed to β-HPV (Fig4.D).
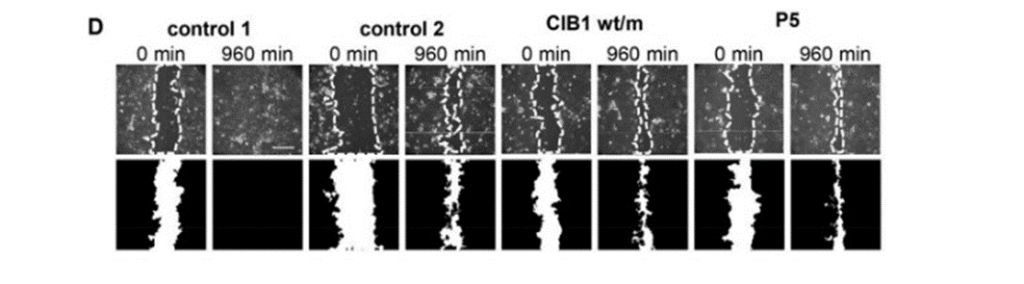
Figure 4: D expresses the results of the wound healing experiment, where there is no difference between the controls (healthy people), the heterozygous for the CIB1 gene, and for CIB1 deficient folks.
Lastly, researchers suggest that CIB1 forms a complex with EVER1 and EVER2, and it is this complex that governs the ability to fend off β-HPV infection. Other HPVs that can infect healthy people all have specific regions of their genome that they can use that β-HPV lacks. Researchers hypothesize that those genes can attack the CIB1-EVER1–EVER2 complex, meaning that other HPVs can infect humans while β-HPV is unable to infect humans unless it encounters someone who is deficient for any of these proteins.
Indeed, there are several main conclusions in this paper. First, that Zn does not play a role in EV. Second, full CIB1 deficiency is an entirely independent and new cause of EV. CIB1 forms a complex with EVER1 and EVER2 that is a restriction factor (which means it prevents and destroys) for β-HPV’s attempts to enter humans. Lastly, the effects of CIB1 deficiency are only displayed when the individual is exposed to β-HPV. More research on EV is necessary, particularly because the disease is not well-known and very rare, in order to more fully explore if Zn still does play a role in the disease, even with the CIB1-EVER1–EVER2 complex acting as a restriction factor, and to deduce the mechanism of the complex to determine if it truly is governing immunity to β-HPV.
Citations:
de Jong, SJ et al. “The Human CIB1-EVER1-EVER2 Complex Governs Keratinocyte-Intrinsic Immunity to β-Papillomaviruses.” The Journal of Experimental Medicine, U.S. National Library of Medicine, Sept. 2018, pubmed.ncbi.nlm.nih.gov/30068544/. Accessed 26 Sept. 2024.
Myers, David J. “Epidermodysplasia Verruciformis.” National Institutes of Health, U.S. National Library of Medicine, 20 July 2024, www.ncbi.nlm.nih.gov/books/NBK534198/. Accessed 26 Sept. 2024.