Since the pioneering treatments employed by Dr. William B. Coley, bacteria have been proposed as a mode of late-stage cancer immunotherapy. Bacteria provide multiple advantages in cancer treatment: direct killing of cancer cells through production toxins, increased host inflammation to help host immune cells kill cancer cells, and effective removal of bacterial treatments through antibiotics following tumor elimination. However, the way which tumor cells and proximal host cells adapt to the presence of bacterial immunotherapy has been understudied.
In the current study, Dr. Kurt Yun Mou and colleagues look to elucidate the mechanisms by which tumor cells circumvent bacteria-mediated cancer killing. They determine the types of proteins expressed by E. Coli when incubated in a mouse tumor, subsequently producing mutant E. Coli which are better equipped to engage cancer cell killing in the presence of host response mechanisms. They observe that iron, an essential nutrient for all living organisms due to its role in oxygen acquisition, is deprived from E. Coli when they are grown in tumors. They then produce mutant E. Coli that can more aggressively acquire the iron that they need to survive.
The authors first compared the protein expression profiles of E. Coli injected into and cultured in mouse colon cancer tumors compared to E. Coli cultured in optimal conditions. They observed that E. Coli cultured in tumors increase expression of iron acquisition mechanisms, including enterobactin, a high-affinity iron-binding protein which bacteria utilize to acquire iron for essential cellular processes. They also observed that host cells highly upregulate bacterial iron acquisition inhibitors. This profiled mouse colon cancer tumors as an iron-deficient environment.
In response, the authors engineered E. Coli which express IroA, a protein which modifies bacterial iron acquisition mechanisms to inhibit host iron deprivation mechanisms. When these E. Coli were inoculated in mice carrying colon, breast, or melanoma tumors, tumor growth was highly inhibited, even resulting in complete remission in 6 mice with colon cancer tumors.
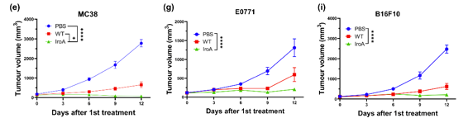
Figure 1. IroA-expressing E. Coli highly reduce tumor growth in colon, breast, and melanoma tumor-carrying mice.
The authors then assessed the protein profile of these IroA E. Coli in comparison to wild-type (WT), meaning non-mutant, E. Coli when each were grown in a mouse colon cancer tumor. They observed that WT E. Coli express far more iron acquisition proteins, while IroA-expressing E. Coli express a protein profile in line with growth and cell maintenance. This provided conformation that IroA E. Coli circumvent host iron deprivation mechanisms when cultured in tumors.
The authors then looked to see if growing IroA-expressing E. Coli in tumors induces infiltration of host immune cells into the tumors, a highly important process in the killing of cancer cells. Following digestion of colon tumors after IroA-E. Coli were injected and cultured, the authors observed that IroA-expressing E. Coli induce increased host immune cell infiltration when compared to WT E. Coli, providing more effective host-mediated cancer cell elimination.
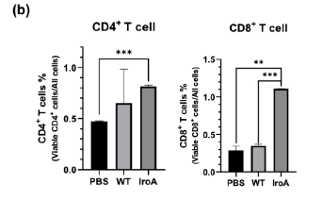
Figure 2. IroA E. Coli induce increased CD4+ and CD8+ T cell infiltration when compared to WT E. Coli.
Following infection with a specific strain of pathogen, a subset of host immune cells will differentiate into a “memory” subset, which initiate a rapid and effective response if the same pathogen re-infects the host. This mechanism can also provide an antitumor response, should a tumor of the same profile reemerge following remission. As a final experiment, the authors rechallenged the mice that achieved complete colon tumor remission following IroA E. Coli treatment with the same cancer, looking to assess whether antitumor immunological memory had been induced. The authors observed that, following rechallenge with the same colon cancer, mice that had previously achieved IroA-mediated tumor remission did not show additional tumor growth. This suggests that IroA E. Coli inoculation induces antitumor immunological memory that protects against future tumors of the same profile (without any further IroA treatment).
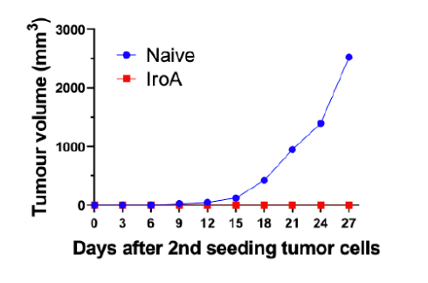
Figure 3. Mice that achieve IroA-mediated tumor remission do not demonstrate additional tumor growth following rechallenge with colon cancer lines.
In conclusion, the current study demonstrates that, in response to bacteria-based immunotherapy, tumor and host cells upregulate iron deprivation mechanisms to kill the invading pathogen. The authors propose a mode by which bacteria can be engineered to circumvent iron deprivation, therefore producing a more effective immunotherapy that even induces antitumor immunological memory. How immunological memory is engaged or whether these engineered bacteria can be removed by typical antibacterial therapeutics is yet to be seen. However, the current study elucidates another mechanism by which cancer evades immunotherapy and an outline for antitumor bacterial optimization.
References:
Huang et al., 2024. Overcoming the nutritional immunity by engineering iron-scavenging bacteria for cancer therapy. eLife 12:RP90798. https://doi.org/10.7554/eLife.90798.3


