Zinc (Zn) is an extremely important mineral and nutrient found throughout our body. It plays many key roles on physiological and molecular scales. On a physiological level Zn plays a role in the immune system, in metabolism, in wound healing, and helps with our sense of taste and smell. On a molecular level, Zn is necessary for the growth and development of cells and plays an important neurosensory role, where it acts as a neuromodulator in synaptic transmissions. Additionally, it regulates the activation of biological molecules and signaling pathways such as transcription factors, enzymes, adapters, or channels. Finally, Zn has an especially crucial role structurally for proteins and DNA, which we will discuss in more detail later on in this post.
Maintaining and adjusting Zn levels in the body is very important for proper biological and cellular processes as well as responses. Our bodies can have intracellular Zn concentrations that reach 1-100µM, but within the cytosol of cells, this concentration is hypothesized to be in the picomolar to low nanomolar range, as Zn is stored in many intracellular compartments and is a multi-functional metal. Our bodies have developed mechanisms to maintain Zn homeostasis, which is carried out by Zn transporters.
There are two categories of Zn transporters: ZIP and ZnT transporters. ZIP proteins are influx transporters that elevate Zn levels by pulling Zn into the cytosol from EC fluid or from intracellular vesicles (Figure 1). ZnT proteins are efflux transporters that reduce cytosolic Zn levels by transporting Zn directly out of the cell or into intracellular compartments (Figure 1). Furthermore, it is important to keep in mind that Zn is not floating around readily, but usually bound to proteins or distributed in vesicles. Some of these proteins include metallothioneins, which are storage proteins for Zn and are essential for homeostasis of this metal. In addition to metallothioneins, there are metallochaperones, which produce a more specific interaction as they deliver Zn to specific target proteins.
Figure 1: Image depicting Zn transport. ZIP proteins are influx transporters and ZnT proteins are efflux transporters.
As highlighted above and in previous slides, Zn homeostasis is very important to maintain in the cells, therefore, making Zn transport essential for proper functioning and survival of cells. Zn transport is critical in physiological events, as highlighted, and is especially important in disease pathogenesis, especially in the development of cancer within the body.
So, what is cancer? Cancer is the uncontrollable division of abnormal cells that invade and spread to other parts of the body. It is caused by endogenous or exogenous factors that cause damage to the genome. Cancer cells need to become immortal, proliferate, and provide themselves nutrients to survive. Without this, the cancer cells will fail and die. This is where Zn plays a crucial role within the cell. Physiological Zn concentrations inhibit cancer cell proliferation and migration, maintain balanced metabolism, and promote apoptosis of cancerous cells or cells that have the potential to become cancerous (i.e. mutated cells).
However, if there is Zinc dyshomeostasis all of these normal functions of the cell are affected, where a Zn Imbalance can lead to cancer cell initiation, causing the division and invasion of cells by other tissues within the body.
Currently, we have been discussing that Zn dyshomeostasis is one of the reasons that lead to cancer, but what specifically is it: the accumulation of or the deficiency of Zn within cells? This figure below (Figure 2), from a review written by Pan and coworkers, demonstrates that a deficiency in Zn, where even a mild deficiency of Zn, can cause cancer. Although this is what is depicted and found in many studies, it is not always the case. Zn has been shown to play a dual role in cancer, and we can see that by taking a closer look at some of the contradictory research in this field.
Figure 2: Graph showing how Zinc concentration when in mild, moderate, or severe deficiency or excess can lead to certain medical disorders. Even a mild deficiency in Zn concentration can cause cancer, as discussed in this article.
In this figure below (Figure 3) from a study done by Abnet et al. (2005), we are looking at esophageal squamous cell carcinoma. Here individuals were assigned different quartiles based on the varying amounts of Zn present in their tissues upon biopsy. Quartile 1 being the lowest amount of Zn and Quartile 4 being the highest amount of tissue zinc levels. Samples were collected over 16 years to measure the survivability of patients with the cancer. When there were lower zinc levels within the cancerous tissue, individuals were less likely to be disease free than those shown to have higher levels of tissue zinc. Zinc levels were measured by collected X-ray fluorescence emission spectra, which produces a spectra with specific peaks that can be traced back to the elements of interest, which in this case were zinc, nickel, copper, and iron.
Figure 3: Graph comparing the percent survivability of patients over the span of 16 years. Individuals that were classified as having higher Zn tissue levels (Quartile 4) compared to those who were classified to have the lowest amount of Zn tissue levels (Quartile 1) were seen to have a higher percentage of survivability over the 16 years that this study was conducted.
In the figure (Figure 4) reproduced from a study done by Christudoss and coworkers (2011), we are looking at tissue zinc levels in three subsets of individuals. From left to right on each bar graph, we have normal individuals, then those with benign prostatic hyperplasia, and then those with prostatic cancer. Zinc levels were measured using atomic absorption spectrophotometry. As seen in these graphs there is a decreased amount of Zn levels within the tissue samples between normal/healthy patients and those with prostate cancer. Urine zinc levels were seen to increase, suggesting that Zn is not being suppressed in the body or its cells. The paper concluded that prostate carcinoma may be associated with reduction in levels of tissue zinc and plasma zinc, and an increase in urine zinc.
Figure 4: Graphs depicting the amount of Zn found in normal, benign prostate hyperplasia, and prostate carcinoma patients.
In the two studies mentioned above, there has been shown to be a deficiency in Zn within cancer cells. Whether this Zn deficiency can be linked to cancer is still unknown as there are contradictory studies. Research conducted by Hashemi et al. (2006) suggests that by depleting Zn within the extracellular matrix surrounding cancer cells as well as depleting Zn in the cytosol of cancer cells, cell viability decreases and apoptosis is induced. In their work, Hashemi and company were comparing the effects of DTPA, a membrane impermeable Zn chelator, and TPEN, a membrane permeable Zn chelator on two different breast cancer cell lines. Measurements were taken every 24 hours for three days. In Panel A of Figure 5, cell viability of cells were measured as Zn chelator (DTPA and TPEN) were increased. In Panel B of Figure 5, the percent of cells that underwent apoptosis was measured as Zn chelator (DTPA and TPEN) were increased. As we can see both DTPA and TPEN significantly decrease cell viability and increase apoptosis within cells.
Figure 5: Measurements of cell viability and percent apoptosis of two breast cancer cell lines as Zn chelator (DTPA and TPEN) concentrations were increased.
As can be seen through these research articles presented, it remains unknown whether a Zn accumulation or Zn deficiency causes cancer. It is important to recognize that these studies were all done in different types of cancer cells, which may suggest that certain cancers may develop from the accumulation of Zn, while others may develop specifically from a Zn deficiency. This may also be more patient specific as well because cancers may vary case by case.
Now that we have discussed Zinc dyshomeostasis and how Zn transport is essential to maintain, we will begin to discuss the essential structural role of Zn within a number of proteins and in the context of cancer. One structural role that Zn has is in the p53 protein, which is known as the “Guardian of the Genome”. The p53 protein has specific functions when it comes to DNA damage response. It activates many pathways to respond to DNA damage, which includes senescence, repairing the DNA, arresting the cell cycle, or inducing apoptosis. In the context of cancer, the p53 protein will arrest the cell cycle or induce apoptosis.
Figure 6: Image depicting how the p53 protein is activated and the downstream pathways that are activated by the p53 protein.
In a study done by Blanden and coworkers (2020) they conduct a biophysical and biochemical analysis of how Zn shapes the folding landscape of the p53 protein and how it can be utilized to reactivate structurally diverse mutants of this protein. They studied the most common p53 mutants that are related to cancer in humans and first classified them into how the mutations affect the function of the protein. This was determined by calculating the amount of free energy released upon binding of the protein when Zn was introduced to the system. They found that mutations could be classified as stability, DNA-contact, and Zn2+-binding class mutations, where the mutation would affect either the overall stability, the affinity for DNA, or the Zn2+-binding domain of the protein, respectively.
Figure 7: Image of the DBD of the p53 protein with its common mutations highlighted. In red are the stability class mutations, blue are the DNA-contact mutations, and in green are the Zn2+-binding mutations. These mutations are also explored in the diagram to the right, where there is also a mixed class of mutations, which means these mutations would affect both the stability as well as the Zn2+-binding domain of the protein.
This figure below (Figure 8) is a heatmap depicting the extent of DNA-binding of various p53 mutants. The KDNA, dissociation constants, for binding of the 22 DBD mutants to a panel of fluorescently labeled oligonucleotides bearing 10 p53 recognition elements was measured. If the mutant DBD shows a higher affinity for the DNA oligonucleotides compared to the WT DBD the heatmap would be more red on the scale. If the mutant DBD shows a lower affinity for the DNA oligonucleotides compared to the WT DBD the heatmap would be more blue on the scale. KDNAvalues were calculated using fluorescence anisotropy. The stability and mixed mutants have similar affinity for DNA compared to the WT, where the E285K mutant shows an increased affinity for DNA which may be due to enhanced electrostatic interactions with the phosphate backbone afforded by negative to positive charge reversal near the active site. When looking at the Zn-binding and DNA-binding mutants there is a decreased affinity seen for the p53 RE. It makes sense why the DNA-binding contact mutant would lose DNA-binding activity, however, the Zn-binding mutants suggest that Zn misligation contributes to loss of DNA binding activity as well. As can be seen in panel B of the second figure, they reconducted the fluorescent anisotropy experiments, using Zn to restore the DNA-binding to the waf1 p53 RE, which all DNA-binding and Zn-binding mutants showed the least affinity for compared to the WT p53 DBD. As can be seen in Figure 8, when Zn is reintroduced to the DNA-binding and Zn-binding mutants of the p53 DBD, DNA binding affinity for certain mutants can be restored. This suggests that Zn not only affects the folding landscape of the p53 protein, but can refold and potentially, in some cases, act as a therapeutic agent to reintroduce the function of certain mutants.
Figure 8: In this figure, there is a heatmap (left) depicting the extent of DNA-binding of various p53 mutant to the 22 DBD mutants to a panel of fluorescently labeled oligonucleotides bearing 10 p53 recognition elements (RE) was measured. The graph (top right) depict anisotropy data measuring the KDNA, dissociation constants, of the various p53 DBD mutants to the waf1 p53 RE. The second graph (bottom right) depicts anisotropy measurements of DNA-binding contact and Zn-binding mutants with and without the introduction of Zn. When Zn was introduced the p53 DBD mutants were stabilized and shown to bind to the waf1 p53 RE, suggesting that Zn reshapes the folding landscape of the protein and can be potentially used as a therapeutic agent to restore function of the protein.
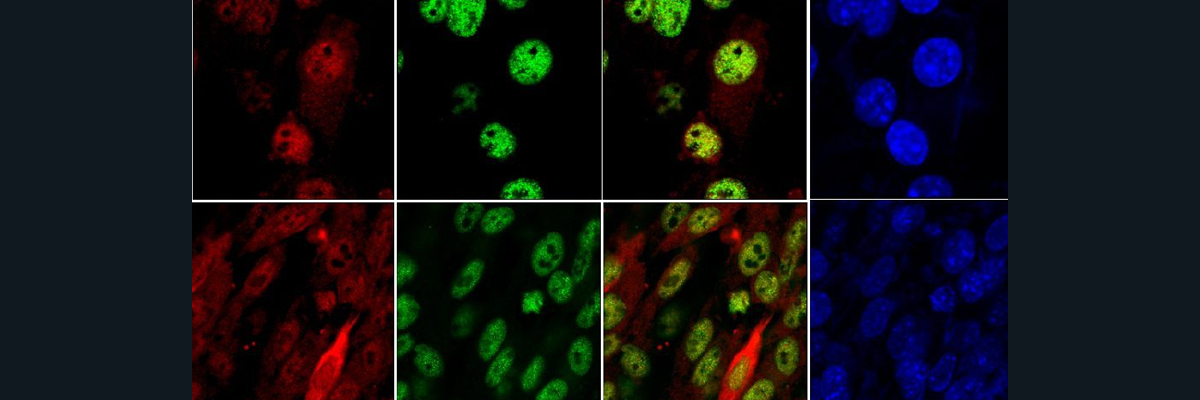
As proposed in the study conducted by Blanden and coworkers (2020), Zn may be potentially used as a therapeutic agent when it comes to restoring the structure and in some cases the function of the p53 DBD. However, there is no free Zn within our body and cells, and so the groups proposes the use of Zn Metallochaperone-1 (ZMC-1) as a therapeutic agent to deliver Zn in a targeted manner to the p53 protein. In their in vitro studies which were conducted in a number of cancer cell lines, Blanden et al. did see a refolding of the mutant p53 proteins of interest. This was measured by PAB240 and PAB1620 staining. Furthermore, in some mutations, ZMC-1 was able to restore the function of the p53 protein as seen by decreased survivability of cancer cells.
Figure 9: Graph demonstrates the relative PAB240 staining for specific p53 mutants, where the light-colored bar is the mutant treated with DMSO and the dark-colored bar is when the p53 mutant is treated with ZMC-1. Western blots were performed to measure the amount of PAB240 staining when mutants were treated with ZMC-1. To see whether there was a restoration of function of the mutant p53 proteins, activation of NOXA and PUMA, proteins in downstream pathways, was measured.
Although this study provides significant information about the p53 protein and how Zn is crucial to its structure and, therefore, its function, this study needs to be further conducted in vivo. This is especially important as Blanden and company suggest that the p53 protein is in equilibrium with its folded and unfolded states at physiological temperature (37ºC). Therefore, it is especially important to further understand the implications of these specific p53 mutations in vivo and how unfolding/misfolding of these mutated proteins at physiological temperatures can be mitigated with the introduction of Zn utilizing ZMC-1. Furthermore, it would be interesting to further conduct studies to understand how ZMC-1 is able to restore the apoptotic and cell-cycle arrest functions of mutant p53 protein in vivo. Which mutants are able to be restored in structure and function with ZMC-1 treatment? Furthermore, it may be interesting to explore the deficiency and excess of zinc in certain cancers and specifically look at the folding and function of the p53 protein in these cases.
Sources:
Christian C. Abnet, Barry Lai, You-Lin Qiao, Stefan Vogt, Xian-Mao Luo, Philip R. Taylor, Zhi-Wei Dong, Steven D. Mark, Sanford M. Dawsey, Zinc Concentration in Esophageal Biopsy Specimens Measured by X-Ray Fluorescence and Esophageal Cancer Risk, JNCI: Journal of the National Cancer Institute, Volume 97, Issue 4, 16 February 2005, Pages 301–306, https://doi.org/10.1093/jnci/dji042 (Figure 3)
Blanden AR, Yu X, Blayney AJ, Demas C, Ha JH, Liu Y, Withers T, CarpizoDR, Loh SN. Zinc shapes the folding landscape of p53 and establishes a pathway for reactivating structurally diverse cancer mutants. Elife. 2020 Dec 2;9:e61487. doi: 10.7554/eLife.61487. PMID: 33263541; PMCID: PMC7728444. (Figures 7-9)
Christudoss P, Selvakumar R, Fleming JJ, Gopalakrishnan G. Zinc status of patients with benign prostatic hyperplasia and prostate carcinoma. Indian J Urol [serial online] 2011 [cited 2022 Apr 5];27:14-8. Available from: https://www.indianjurol.com/text.asp?2011/27/1/14/78405 (Figure 4)
Mohammad Hashemi, Saeid Ghavami, Mehdi Eshraghi, Evan P. Booy, Marek Los, Cytotoxic effects of intra and extracellular zinc chelation on human breast cancer cells, European Journal of Pharmacology, Volume 557, Issue 1,2007, Pages 9-19, ISSN 0014-2999, https://doi.org/10.1016/j.ejphar.2006.11.010. (Figure 5)
Moulder, D.E.; Hatoum, D.; Tay, E.; Lin, Y.; McGowan, E.M. The Roles of p53 in Mitochondrial Dynamics and Cancer Metabolism: The Pendulum between Survival and Death in Breast Cancer? Cancers 2018, 10, 189. https://doi.org/10.3390/cancers10060189 (Figure 6)
Pan, Z., Choi, S., Ouadid-Ahidouch, H., Yang, J. M., Beattie, J. H., & Korichneva, I. (2017). Zinc transporters and dysregulated channels in cancers. Frontiers in bioscience (Landmark edition), 22(4), 623–643. https://doi.org/10.2741/4507 (Figure 2)
Wang, J., Zhao, H., Xu, Z., & Cheng, X. (2020). Zinc dysregulation in cancers and its potential as a therapeutic target. Cancer biology & medicine, 17(3), 612–625. https://doi.org/10.20892/j.issn.2095-3941.2020.0106 (Figure 1)