Parkinson’s Disease (PD) is the second most common neurodegenerative disease after Alzheimer’s disease, affecting about 1-2 people 1000, including about 1% of people over 60. There are genetic markers in 5-10% of patients, but there is no direct connection as many patients develop symptoms without these markers. Symptoms of PD include difficulty walking and talking, tremor, and stiffness. Similar to Alzheimer’s Disease, Parkinson’s has an early onset type, but only affects about 5-10% of patients, otherwise, patients tend to be over 60. Parkinson’s occurs when nerve cells in the basal ganglia (which controls movement) become impaired or die. These cells produce less dopamine and norepinephrine, which causes problems with movement, but unclear exactly what causes this to happen. Lewy bodies, clumps of 𝛼synuclein (𝛼Syn) protein develop, causing increased damage to the brain. Treatments for PD include drugs that increase dopamine (levodopa and carbidopa), MAO-B inhibitors, amantadine, anticholinergic medications, and deep brain stimulation.
Metals play an important role in the development of PD, as Cu(II) and other metals bind to the alpha-synuclein proteins that cause Parkinson’s. These metal ions, especially Cu(II) increase this aggregation. As a result of copper being trapped in these proteins, copper deficiency is common in Parkinson’s disease, especially in the substantia nigra, which is where dopamine is produced.
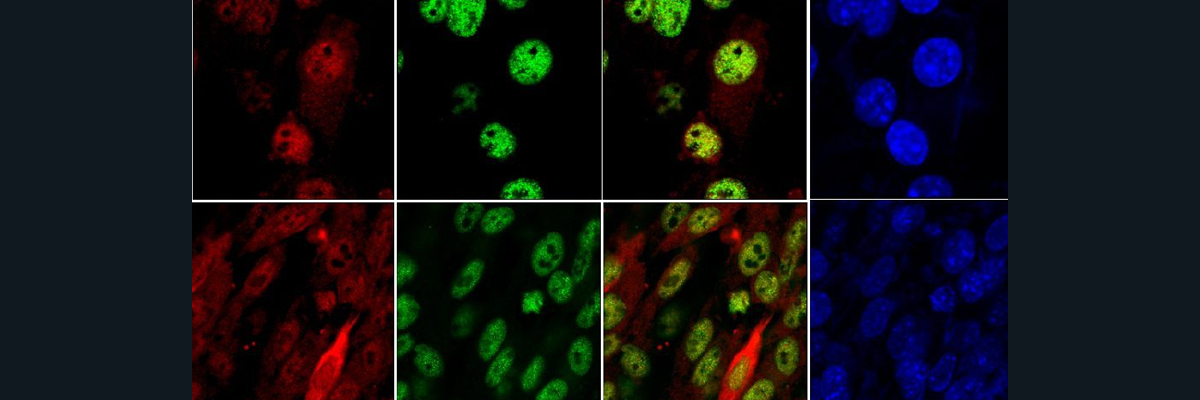
As a result of these mechanisms, researchers from Chambers University and the University of Gothenburg wanted to see if the introduction of the copper chaperone Atox1 would inhibit amyloid formation 𝛼Syn. They used NMR to study the structure of the complex formed between Atox1 and 𝛼Syn. They used a plasmid called pRK172 to produce 𝛼Syn, labeled it using specific isotopes that would be visible in NMR, and purified it. They then did the same thing with Atox1 using a different plasmid. The researchers then analyzed NMR data from 13C and 15N in order to construct the structure of the protein. They looked at the NMR shifts when starting with Atox1 and when starting with 𝛼Syn. The researchers found a structure for the protein and then used PLA to confirm the interactions between the various proteins. After analyzing the structure of the Atox1-Cu-𝛼Syn complex, the researchers proposed a possible way that this complex influences PD. In normal conditions, they proposed that Cu-Atox1 interacts directly with 𝛼Syn, which stops the possible development of a pathogenic form of 𝛼Syn. When there are low levels of Cu, this interaction does not occur because Atox1 and 𝛼Syn cannot interact without Cu. As a result, it is possible for the 𝛼Syn to collect, but usually not super fast because the copper is not there to make the aggregates form fast. However, when the cell is under oxidative stress, Cu coordinating cysteines form a disulfide bond which causes Atox1 not to bind copper. This leads to a higher concentration of copper in the cells, and amalgamation at faster rates.
Read the paper here:
Horvath, Istvan, Stéphanie Blockhuys, Darius Šulskis, Stellan Holgersson, Ranjeet Kumar, Björn M. Burmann, and Pernilla Wittung-Stafshede Interaction between Copper Chaperone Atox1 and Parkinson’s Disease Protein α-Synuclein Includes Metal-Binding Sites and Occurs in Living Cells ACS Chemical Neuroscience 2019 10 (11), 4659-4668, DOI: 10.1021/acschemneuro.9b00476